Digestive System
Subtopic:
Liver Cirrhosis
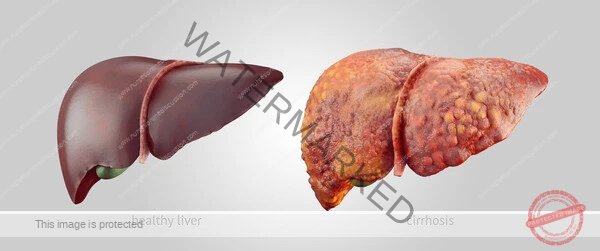
Liver cirrhosis represents the advanced stage of chronic liver disease, marked by extensive scarring (fibrosis) that disrupts the normal structure and function of the liver. This irreversible process leads to the formation of abnormal nodules within the liver tissue, fundamentally altering its architecture and impeding blood flow. As the culmination of various forms of chronic liver injury, cirrhosis significantly compromises the liver’s ability to perform its vital roles, resulting in substantial health complications and increased mortality. It stands as a major challenge in global healthcare.
Definition and Characteristics
Cirrhosis is defined by two main pathological features:
Fibrosis: Excessive deposition of extracellular matrix proteins, particularly collagen, leading to scarring of the liver tissue. This replaces healthy liver parenchyma.
Nodular Regeneration: Formation of disorganized nodules of regenerating hepatocytes, often surrounded by fibrous septa. These nodules lack the normal lobular architecture and disrupt blood flow.
These changes result in:
Impaired Liver Function: Reduced ability of hepatocytes to perform their metabolic, synthetic, and detoxifying functions.
Portal Hypertension: Increased pressure in the portal venous system due to resistance to blood flow through the fibrotic and nodular liver.
Cirrhosis can be classified histologically (e.g., micronodular, macronodular) or clinically based on the presence or absence of complications (compensated vs. decompensated).
Compensated Cirrhosis: The liver is significantly damaged but can still perform most of its essential functions. Patients may be asymptomatic or have non-specific symptoms. The prognosis is relatively better, but complications can develop.
Decompensated Cirrhosis: The liver’s function is severely impaired, leading to the development of major complications of portal hypertension and hepatocellular dysfunction (e.g., ascites, hepatic encephalopathy, variceal bleeding, jaundice). This stage is associated with a poor prognosis.
Etiology and Causes
Numerous chronic liver diseases can lead to cirrhosis. The most common causes vary geographically but include:
Chronic Viral Hepatitis:
Hepatitis C (HCV): A leading cause of cirrhosis and hepatocellular carcinoma (HCC) worldwide. Often progresses slowly and silently over decades.
Hepatitis B (HBV): Another major cause globally. Can be acquired perinatally or in adulthood. Chronic infection can lead to cirrhosis and HCC. Hepatitis D (HDV) infection in individuals with HBV can accelerate the progression to cirrhosis.
Alcoholic Liver Disease (ALD):
Results from prolonged excessive alcohol consumption.
Ranges from alcoholic fatty liver to alcoholic hepatitis and ultimately cirrhosis.
The amount and duration of alcohol intake are key determinants.
Non-Alcoholic Fatty Liver Disease (NAFLD) / Non-Alcoholic Steatohepatitis (NASH):
Associated with metabolic risk factors such as obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome.
NAFLD is characterized by fat accumulation in the liver. NASH is a more severe form involving inflammation and hepatocyte injury, which can progress to fibrosis, cirrhosis, and HCC.
Increasingly recognized as a major cause of cirrhosis globally, particularly in Western countries.
Chronic Biliary Diseases:
Conditions that cause chronic obstruction or inflammation of the bile ducts, leading to cholestasis (impaired bile flow) and subsequent liver damage and fibrosis.
Primary Biliary Cholangitis (PBC): An autoimmune disease affecting the small intrahepatic bile ducts.
Primary Sclerosing Cholangitis (PSC): A chronic inflammatory condition causing strictures and fibrosis of the bile ducts (intrahepatic and/or extrahepatic). Often associated with inflammatory bowel disease.
Secondary Biliary Cirrhosis: Caused by prolonged obstruction of the extrahepatic bile ducts (e.g., by gallstones, strictures, tumors).
Genetic and Metabolic Disorders:
Hemochromatosis: A genetic disorder causing excessive iron absorption and deposition in the liver and other organs.
Wilson’s Disease: A genetic disorder causing impaired copper metabolism and accumulation in the liver, brain, and other tissues.
Alpha-1 Antitrypsin Deficiency: A genetic disorder leading to reduced levels of alpha-1 antitrypsin, a protein that protects the lungs and liver from damage.
Glycogen Storage Diseases and other metabolic disorders.
Autoimmune Hepatitis:
A chronic inflammatory liver disease caused by the body’s immune system attacking the hepatocytes.
Drugs and Toxins:
Certain medications (e.g., methotrexate, isoniazid, amiodarone) and toxins can cause chronic liver injury leading to cirrhosis.
Vascular Disorders:
Budd-Chiari Syndrome: Obstruction of the hepatic veins, leading to congestion and damage.
Chronic Heart Failure: Severe, prolonged right-sided heart failure can lead to “cardiac cirrhosis” due to chronic passive congestion of the liver.
Cryptogenic Cirrhosis:
Cirrhosis of unknown cause after thorough investigation. A significant proportion of these cases are now believed to be due to undiagnosed NAFLD/NASH.
Pathophysiology
The development of cirrhosis is a complex process involving chronic injury, inflammation, fibrosis, and regeneration.
Chronic Liver Injury: The initial insult (e.g., virus, alcohol, metabolic factors) causes damage to hepatocytes.
Inflammation: Damaged hepatocytes release signals that attract inflammatory cells (e.g., lymphocytes, macrophages). This chronic inflammation perpetuates liver injury.
Activation of Hepatic Stellate Cells: Hepatic stellate cells, normally quiescent cells in the space of Disse (between hepatocytes and sinusoids), become activated in response to inflammatory mediators and growth factors. Activated stellate cells transform into myofibroblast-like cells.
Fibrosis: Activated stellate cells are the primary source of excessive extracellular matrix proteins, particularly collagen types I and III. This leads to the deposition of fibrous tissue in the liver, forming septa that connect portal tracts and central veins.
Disruption of Architecture and Nodular Regeneration: The fibrous septa and ongoing hepatocyte injury disrupt the normal lobular architecture. Hepatocytes attempt to regenerate, but the regeneration is disorganized, leading to the formation of nodules surrounded by fibrous tissue. This further distorts the liver structure.
Portal Hypertension: The distorted architecture and fibrous tissue increase resistance to blood flow through the liver, leading to increased pressure in the portal vein. This portal hypertension is a major driver of many of the complications of cirrhosis. Mechanisms include:
Increased intrahepatic resistance (due to fibrosis, nodules, and contraction of activated stellate cells and myofibroblasts).
Increased splanchnic blood flow (vasodilation in the mesenteric circulation).
Hepatocellular Dysfunction: The reduced number of functional hepatocytes and the disrupted architecture impair the liver’s ability to perform its metabolic, synthetic, and detoxifying functions. This leads to:
Reduced synthesis of proteins (e.g., albumin, clotting factors).
Impaired detoxification of waste products (e.g., ammonia).
Impaired metabolism of hormones and drugs.
Reduced bile formation and excretion.
Clinical Manifestations
The clinical presentation of cirrhosis varies depending on whether it is compensated or decompensated.
Compensated Cirrhosis:
May be asymptomatic.
Non-specific symptoms may include fatigue, weakness, loss of appetite, and weight loss.
Physical examination may be normal or show subtle findings like spider angiomas or palmar erythema.
Decompensated Cirrhosis: The development of complications indicates decompensation. These include:
Ascites: Accumulation of excess fluid in the peritoneal cavity. Caused by portal hypertension, reduced albumin synthesis, and impaired renal sodium and water excretion.
Variceal Bleeding: Bleeding from varices (enlarged veins) in the esophagus or stomach, which develop as collateral vessels to decompress the portal system due to portal hypertension. Can be life-threatening.
Hepatic Encephalopathy: A spectrum of neuropsychiatric abnormalities ranging from subtle cognitive impairment to coma. Caused by the accumulation of neurotoxins, particularly ammonia, that are not adequately cleared by the impaired liver.
Jaundice: Yellowing of the skin and sclerae due to impaired bilirubin excretion.
Spontaneous Bacterial Peritonitis (SBP): Infection of the ascitic fluid, often without an obvious source. A serious complication in patients with ascites.
Hepatorenal Syndrome: Renal failure that occurs in patients with advanced cirrhosis and ascites in the absence of primary kidney disease.
Hepatopulmonary Syndrome: A triad of liver disease, intrapulmonary vascular dilatations, and arterial hypoxemia.
Portopulmonary Hypertension: Pulmonary hypertension associated with portal hypertension.
Coagulopathy: Impaired synthesis of clotting factors by the liver, leading to increased risk of bleeding.
Splenomegaly: Enlargement of the spleen due to portal hypertension, which can lead to thrombocytopenia (low platelet count) and leukopenia (low white blood cell count).
Other signs of chronic liver disease: Spider angiomas, palmar erythema, gynecomastia (in men), testicular atrophy, loss of libido, muscle wasting, nail changes (e.g., Terry’s nails), easy bruising.
Diagnosis
Diagnosing cirrhosis involves a combination of clinical evaluation, laboratory tests, imaging studies, and sometimes liver biopsy.
Medical History and Physical Examination: Identify risk factors, symptoms, and signs of liver disease and its complications.
Laboratory Tests:
Liver Function Tests (LFTs): Bilirubin (often elevated), albumin (often decreased), ALT and AST (may be normal, elevated, or decreased in advanced disease), ALP and GGT (may be elevated, especially in biliary causes).
Prothrombin Time (PT) / International Normalized Ratio (INR): Often prolonged in advanced cirrhosis due to impaired synthesis of clotting factors. This is a sensitive marker of liver synthetic function and prognosis.
Complete Blood Count (CBC): May show anemia, thrombocytopenia, and leukopenia (due to splenomegaly or other factors).
Kidney Function Tests (Urea, Creatinine, Electrolytes): To assess renal function, especially if hepatorenal syndrome is suspected.
Serological Tests: For viral hepatitis (HBV, HCV), autoimmune markers, etc., to identify the underlying cause.
Tests for Metabolic/Genetic Disorders: Iron studies (hemochromatosis), ceruloplasmin and urinary copper (Wilson’s disease), alpha-1 antitrypsin levels.
Alpha-fetoprotein (AFP): A tumor marker that may be elevated in hepatocellular carcinoma. Used for surveillance in patients with cirrhosis.
Imaging Studies:
Abdominal Ultrasound: Can show changes in liver size and texture (often nodular), splenomegaly, ascites, and dilated portal vein. Doppler ultrasound can assess portal vein flow.
Computed Tomography (CT) Scan and Magnetic Resonance Imaging (MRI): Provide more detailed images of the liver and surrounding organs, useful for assessing the degree of cirrhosis, detecting HCC, and evaluating for complications.
Transient Elastography (FibroScan): A non-invasive method that measures liver stiffness, which correlates with the degree of fibrosis. A high stiffness value suggests cirrhosis.
Magnetic Resonance Elastography (MRE): Another non-invasive imaging technique for assessing liver stiffness.
Endoscopy: Upper endoscopy is performed to screen for and manage esophageal and gastric varices in patients with cirrhosis.
Liver Biopsy:
Historically the gold standard for diagnosing cirrhosis.
Provides histological confirmation of fibrosis and nodular regeneration and can help identify the underlying cause.
Less frequently required for diagnosis now with the advent of non-invasive methods, but may still be necessary in cases where the diagnosis is uncertain or to assess the severity and activity of the underlying disease.
Management
Management of cirrhosis focuses on:
Treating the Underlying Cause: This is crucial to halt or slow the progression of liver damage. Examples include antiviral therapy for chronic viral hepatitis, abstinence from alcohol for ALD, weight loss and lifestyle modifications for NAFLD/NASH, immunosuppression for autoimmune hepatitis, and specific treatments for metabolic disorders.
Preventing and Managing Complications:
Variceal Bleeding: Primary prophylaxis with non-selective beta-blockers or endoscopic variceal ligation (EVL) in patients with high-risk varices. Acute bleeding requires urgent endoscopic hemostasis, vasoactive drugs (e.g., octreotide), and antibiotics. Secondary prophylaxis after bleeding involves a combination of beta-blockers and EVL.
Ascites: Sodium restriction, diuretics (spironolactone and furosemide). Large volume paracentesis for tense ascites. Transjugular intrahepatic portosystemic shunt (TIPS) may be considered for refractory ascites.
Hepatic Encephalopathy: Identification and treatment of precipitating factors (e.g., infection, bleeding, constipation, certain medications). Lactulose (to reduce ammonia absorption) and rifaximin (a non-absorbable antibiotic to reduce ammonia-producing bacteria) are the mainstays of treatment.
Spontaneous Bacterial Peritonitis (SBP): Empirical antibiotics (e.g., cefotaxime) followed by culture-directed therapy. Prophylactic antibiotics may be indicated in high-risk patients.
Hepatorenal Syndrome: Management involves addressing precipitating factors, albumin infusion, and vasoconstrictors (e.g., terlipressin). Liver transplantation is the definitive treatment.
Hepatocellular Carcinoma (HCC): Regular surveillance with ultrasound and AFP in patients with cirrhosis allows for early detection and potentially curative treatment (e.g., resection, transplantation, locoregional therapy).
Nutritional Support: Addressing malnutrition, which is common in advanced cirrhosis.
Liver Transplantation: The definitive treatment for decompensated cirrhosis and early-stage HCC in suitable candidates. It replaces the diseased liver with a healthy donor liver.
Prognosis
The prognosis of cirrhosis depends on the underlying cause, the severity of liver dysfunction, and the presence and severity of complications. Prognostic models, such as the Child-Pugh score and the MELD (Model for End-Stage Liver Disease) score, are used to assess the severity of cirrhosis and predict survival. Compensated cirrhosis has a better prognosis than decompensated cirrhosis. The development of complications significantly worsens the prognosis. Liver transplantation offers a chance for long-term survival in patients with decompensated cirrhosis.
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved Design & Developed by Opensigma.co

