Managing Children Living with HIV/AIDS
Subtopic:
Prevention and Control of HIV/AIDS
Prevention Framework in children and infants.
Prevention in Pediatrics
∙ Behavioral change and risk reduction interventions
∙ Biomedical prevention interventions
∙ Structural intervention
BEHAVIORAL CHANGE AND RISK REDUCTION INTERVENTIONS
The main focus of behavioral interventions is to encourage young people to start having sex later, promote safer sexual practices, reduce the number of sexual partners (especially having multiple partners at the same time), and discourage sexual relationships involving large age gaps or where sex is exchanged for something.
Types of behavioral change
∙ Service delivery
∙ Risk assessment for client
∙ Provide socio-behavioral change Communication (SBCC) and link to services as appropriate
∙ Condom promotion and provision
Service delivery
The Ugandan government works to ensure that:
1. ⇒ Each health facility/program should have a focal person for HIV prevention: Every health service location should have a designated individual responsible for HIV prevention efforts.
2. ⇒ All staff offering prevention services need to be trained: Personnel providing prevention services must receive proper training.
3. ⇒ Outreaches for key and priority populations: Prevention programs should actively reach out to specific groups who are at higher risk of HIV.
Risk assessment
4. ⇒ Offer HTS to sexually active adolescents, pregnant mothers who have not tested in the last 12 months or have had unprotected sex in last three months: HIV testing services should be offered to teenagers who are sexually active and to pregnant women who haven’t been tested recently or have engaged in risky sexual behavior.
5. ⇒ HIV testing for infants born of HIV infected mothers: Babies born to mothers with HIV should be tested for the virus.
6. ⇒ Assess sexual behavior of the in pregnant mothers and adolescents (ask if condoms are used, frequency, the number of partners, transactional sex/sex work) and if the client is involved in transactional sex/sex work encourage correct and consistent condom use: Healthcare providers should ask pregnant women and adolescents about their sexual practices, including condom use, frequency of sex, number of partners, and involvement in sex work, and strongly advise consistent condom use if they are involved in transactional sex.
Provide socio-behavioral change Communication (SBCC) and link to services as appropriate
7. ⇒ Discuss delay of onset of sexual debut in children and adolescents (abstinence) ⇒ Discuss correct and consistent condom use and offer condoms as appropriate to adolescents ⇒ Discourage multiple, concurrent sexual partnerships to promote faithfulness with a partner of known status: Educate children and adolescents about the benefits of delaying sexual activity, teach them how to use condoms correctly and consistently (and provide condoms), and advise against having multiple sexual partners at the same time, encouraging commitment to one partner whose HIV status is known.
8. ⇒ Discuss with the adolescents about sexual and reproductive health services and link to services as appropriate: Talk to teenagers about available sexual and reproductive health services and help them access these services.
9. ⇒ Discourage risky cultural practices such as childhood marriages: Advise against harmful traditional practices like marrying children.
10. ⇒ Identify, refer and link clients to other available facility and community programs: Connect individuals with other relevant health and support programs in the clinic and community.
11. ⇒ Assess for violence, (physical, emotional, or sexual); if child discloses sexual violence, assess if the client was raped and act immediately: Check for signs of any form of violence; if a child reveals sexual violence, immediately assess if they were raped and take appropriate action.
Condom promotion and provision
12. ⇒ Discuss condom use as an option for risk reduction in pregnant mothers and adolescent ∙ Discuss barriers to condom use to pregnant mothers and adolescent: Talk about using condoms as a way to reduce risk for pregnant women and teenagers and discuss the reasons why they might not be using condoms.
13. ⇒ Clarify any questions and dispel myths around condoms: Answer any questions and correct any false beliefs people might have about condoms.
Biomedical prevention interventions
The main medical interventions to prevent HIV include:
∙ EMTCT (Elimination of Mother-to-Child Transmission)
∙ Safe male circumcision (SMC)
∙ ART (Antiretroviral Therapy)
∙ PEP (Post-Exposure Prophylaxis)
∙ PrEP (Pre-Exposure Prophylaxis)
∙ Blood transfusion safety
∙ STI screening and treatment (Screening and treating sexually transmitted infections)
Safe male circumcision (SMC)
Male circumcision is a surgical procedure to remove the foreskin from the penis. SMC can lower the risk of HIV transmission to men (including adolescent boys) who are circumcised by about 60%.
Blood transfusion safety
This involves:
Ensuring the screening of blood donors for HIV and hepatitis B: Making sure that donated blood is tested for HIV and hepatitis B before use.
Ensuring proper storage and administration: Storing and giving blood transfusions in a safe and correct manner.
STI screening and treatment
This means:
Integration of STI services in all health programs e.g. YCC, MCH: Making sure that services for diagnosing and treating sexually transmitted infections are part of routine health programs, such as Youth Corners and Maternal and Child Health clinics.
EMTCT (Elimination of Mother-to-Child Transmission of HIV)
These are:
Measures of reducing the risk of HIV transmission to the child during pregnancy, labor, puerperium and breastfeeding: Steps taken to lower the chances of a mother with HIV passing the virus to her baby during pregnancy, childbirth, the period after delivery, and while breastfeeding.
Post-exposure prophylaxis (PEP)
Post-exposure prophylaxis (PEP) is the short-term use of ARVs to reduce the likelihood of acquiring HIV infection after potential occupational or non-occupational exposure: PEP involves taking antiretroviral medications for a short period to try and prevent HIV infection after a possible exposure to the virus at work or in other situations.
Types of exposure:
1. ∙ Occupational exposures occur in the health care or laboratory setting and include sharps and needlestick injuries or splashes of body fluids to the skin and mucous membranes: Exposure at work in healthcare or lab settings, like getting stuck with a needle or having body fluids splash onto the skin or into the eyes or mouth.
2. Non-occupational exposures include unprotected sex, exposure following assault like in rape and defilement, and road traffic accidents: Exposure outside of work, such as having sex without a condom, after a sexual assault, or in accidents involving blood.
Steps for providing Post Exposure Prophylaxis
Step 1: Clinical assessment and providing first aid
Conduct a rapid assessment of the client to assess exposure and risk and provide immediate care: Quickly check the person to understand the type of exposure and the risk involved, and give immediate first aid.
Occupational exposure:
After a needlestick or sharp injury
∙ Do not squeeze or rub the injury site: Avoid pressing or rubbing the wound.
∙ Wash the site immediately with soap or mild disinfectant (chlorhexidine gluconate solution): Clean the wound right away with soap and water or a mild disinfectant.
∙ Use antiseptic hand rub/gel if no running water: If there is no running water, use an antiseptic hand rub.
∙ Don’t use strong, irritating antiseptics (like bleach or iodine): Avoid using harsh antiseptics like bleach or iodine.
After a splash of blood or body fluids in contact with intact skin
∙ Wash the area immediately: Wash the affected skin right away.
∙ Use antiseptic hand rub/gel if no running water: If running water isn’t available, use an antiseptic hand rub.
∙ Don’t use strong, irritating antiseptics (like bleach or iodine): Avoid using strong antiseptics.
Step 2: Eligibility assessment
Provide PEP when:
∙ Exposure occurred within the past 72 hours: The exposure happened within the last three days.
∙ The exposed individual is not infected with HIV: The person exposed is HIV negative.
∙ The ‘source’ is HIV-infected, has unknown HIV status or is high risk: The person the exposure came from has HIV, their HIV status is unknown, or they are at high risk of having HIV.
Do not provide PEP when:
∙ The exposed individual is already HIV-positive: The person exposed already has HIV.
∙ The source is established to be HIV-negative: It’s confirmed that the source of the exposure does not have HIV.
∙ Individual was exposed to bodily fluids that do not pose a significant risk (e.g. tears, non-blood stained saliva, urine, sweat): The exposure was to fluids that are very unlikely to transmit HIV.
∙ Exposed individual declines an HIV test: The person who was exposed does not want to get tested for HIV.
Step 3: Counseling and support
Counsel on:
∙ The risk of HIV from the exposure: Explain the actual risk of getting HIV from the specific exposure.
∙ Risks and benefits of PEP: Discuss the potential advantages and disadvantages of taking PEP medication.
∙ Side effects of ARVs: Inform them about the possible side effects of the antiretroviral drugs used in PEP.
∙ Enhanced adherence if PEP is prescribed: Stress the importance of taking PEP medication exactly as prescribed.
∙ Importance of linkage for further support for sexual assault cases: For cases of sexual assault, emphasize the need to connect with support services.
Step 4: Prescription
∙ PEP should be started as early as possible, not beyond 72 hours of exposure: It’s best to start PEP as soon as possible after exposure, and it’s generally not recommended if more than 72 hours have passed.
∙ Recommended regimens include:
⇒ Pregnant mothers/adults: TDF+3TC+ATV/r: A common PEP combination for pregnant women and adults includes the drugs tenofovir disoproxil fumarate (TDF), lamivudine (3TC), and atazanavir boosted with ritonavir (ATV/r).
⇒ Children: ABC+3TC+LPV/r: For children, a typical PEP combination is abacavir (ABC), lamivudine (3TC), and lopinavir boosted with ritonavir (LPV/r).
∙ A complete course of PEP should run for 28 days: The full course of PEP treatment lasts for 28 days.
∙ Do not delay the first doses because of lack of baseline HIV test: Do not wait for the results of an initial HIV test before starting the first doses of PEP.
∙ Document the event and patient management in the PEP register (ensure confidentiality of patient data): Record the details of the exposure and the care given in a confidential PEP register.
Step 5: Provide follow-up
∙ Discontinue PEP after 28 days: Stop taking PEP medication after completing the 28-day course.
∙ Perform follow-up HIV testing three months after exposure: Get tested for HIV again three months after the exposure.
∙ Counsel and link to HIV clinic for care and treatment if HIV-positive: If the follow-up test is positive, provide counseling and connect the person to an HIV clinic for ongoing care and treatment.
∙ Provide prevention and education/risk reduction counseling if HIV-negative: If the follow-up test is negative, offer advice on how to prevent future HIV exposure.
ORAL PRE-EXPOSURE PROPHYLAXIS (PrEP)
PrEP is the use of ARV drugs by people who are not infected with HIV to block the acquisition of HIV: PrEP involves HIV-negative individuals taking antiretroviral medication to prevent them from getting infected with HIV if they are exposed.
The process of providing pre-exposure prophylaxis (PrEP)
1. ∙ Eligibility for PrEP
2. ∙ Screening for PrEP eligibility
3. ∙ Steps to initiation of PrEP
4. ∙ Follow-up/ monitoring clients on PrEP
5. ∙ Guidance on discontinuing PrEP
Step 1: Eligibility for PrEP
PrEP provides an effective additional biomedical prevention option for HIV-negative people at substantial risk of acquiring HIV infection. These include people who:
∙ Have multiple sexual partners
∙ Engage in transactional sex including sex workers
∙ Use or abuse injectable drugs and alcohol
∙ Have had more than one episode of an STIwithin the last twelve months
∙ Are part of a discordant couple, especially if the HIV-positive partner is not on ART or has been on ART for less than six months: This refers to couples where one partner has HIV and the other does not, particularly if the HIV-positive partner is not on treatment or has recently started.
∙ Are recurrent users of PEP (3 consecutive cycles of PEP)
∙ Engage in anal sex
These risk factors are likely to be more prevalent in populations such as sex workers, fisher folk, long distance truck drivers, men who have sex with men (MSM), uniformed forces, and adolescents and young women engaged in transactional sex: These groups are more likely to have several of the risk factors listed above.
Step 2; Screening for PrEP eligibility
After meeting the eligibility criteria:
∙ Confirm HIV-negative status: Make sure the person is HIV-negative before starting PrEP.
∙ Rule out acute HIV infection: Check to ensure they don’t have a very recent HIV infection.
∙ Assess for hepatitis B infection: if negative, patient is eligible for PrEP; if positive, refer patient for management: Check for hepatitis B; if negative, they can start PrEP; if positive, they need to be referred for hepatitis B care.
∙ Assess for contraindications to TDF/FTC: Check if there are any medical reasons why they shouldn’t take the PrEP medication (tenofovir disoproxil fumarate/emtricitabine).
Step 3: Steps to initiation of PrEP
Provide risk-reduction and PrEP medication adherence counseling: Offer advice on safer sex practices and explain how important it is to take PrEP medication as directed.
∙ Provide condoms and education on their use: Give condoms and instructions on how to use them correctly.
∙ Initiate a medication adherence plan: Help the person create a plan to remember to take their PrEP medication daily.
∙ Prescribe a once-daily pill of TDF (300mg) and FTC (200mg): The standard PrEP medication is a single pill containing 300mg of tenofovir disoproxil fumarate (TDF) and 200mg of emtricitabine (FTC), taken once a day.
∙ Initially, provide a 1-month TDF/FTC prescription (1 tablet orally, daily) together with a 1-month follow-up date: At the start, give a prescription for one month’s worth of PrEP and schedule a follow-up appointment in one month.
∙ Counsel client on side effects of TDF/FTC: Inform the person about potential side effects of the PrEP medication.
Step 4: Follow-up/ monitoring clients on PrEP
∙ After the initial visit, the patient should be given a two-month follow-up appointment and thereafter quarterly appointments: After the first follow-up, schedule the next appointment for two months later, and then every three months after that.
∙ Perform an HIV antibody test every three months: Test for HIV every three months.
∙ For women, perform a pregnancy test based on clinical history: Offer pregnancy tests to women as needed, based on their situation.
∙ Review the patient’s understanding of PrEP, any barriers to adherence, tolerance to the medication as well as any side effects: Discuss how well they understand PrEP, any reasons they might be missing doses, how well they are tolerating the medication, and any side effects they are experiencing.
∙ Review the patient’s risk exposure profile and perform risk-reduction counseling: Talk about their ongoing risk of HIV exposure and reinforce advice on how to stay safe.
∙ Evaluate and support PrEP adherence at each clinic visit: Check how well they are taking their PrEP medication at every visit and offer support to help them stick to the schedule.
∙ Evaluate the patient for any symptoms of STIs at every visit and treat as needed: Check for any signs of sexually transmitted infections at each visit and provide treatment if necessary.
Step 5: Guidance on discontinuing PrEP
∙ Acquisition of HIV infection: If the person becomes HIV-positive.
∙ Changed life situations resulting in lowered risk of HIV acquisition: If their circumstances change and they are no longer at high risk.
∙ Intolerable toxicities and side effects: If they experience severe side effects from the medication.
∙ Chronic non-adherence to the prescribed dosing regimen despite efforts to improve daily pill-taking: If they consistently fail to take the medication as directed, even with support.
∙ Personal choice
∙ HIV-negative in a sero-discordant relationship when the positive partner has achieved sustained viral load suppression (condoms should still be used consistently: If the HIV-negative person is in a relationship with an HIV-positive partner whose virus is fully suppressed by treatment (but condoms are still recommended for extra protection).
MOTHER-TO-CHILD TRANSMISSION OF HIV
Approximately one-third of the women who are infected with HIV can pass it to their babies: Around 30% of women with HIV can transmit the virus to their children.
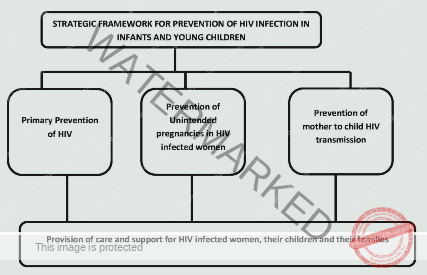
Elements of elimination of mother to child transmission
1. ∙ : Primary prevention of HIV infection Women and men of reproductive age including adolescents: Preventing HIV infection in the first place among all adults and teenagers who can have children.
2. ∙ : Prevention of unintended pregnancies among women living with HIV Women including adolescents living with HIV and their partners: Helping women with HIV avoid getting pregnant if they don’t want to.
3. ∙ : Prevention of HIV transmission from women living with HIV to their infants Pregnant and breastfeeding women including adolescents living with HIV: Taking steps to stop mothers with HIV from passing the virus to their babies during pregnancy, childbirth, and breastfeeding.
4. ∙: Provision of treatment, care, and support to women infected with HIV, their children and their families Women living with HIV and their families: Providing medical care, support, and other assistance to women with HIV, their children, and their families.
Cause
Time of transmission;
∙ During pregnancy (15-20%)
∙ During time of labour and delivery (60%-70%)
∙ After delivery through breast feeding (15%-20%)
Pre-disposing factors
∙ High maternal viral load: When the mother has a large amount of HIV in her blood.
∙ Depleted maternal immunity (e.g. very low CD4 count): When the mother’s immune system is very weak (indicated by a low CD4 count).
∙ Prolonged rupture of membranes: When the amniotic sac breaks for a long time before delivery.
∙ Intra-partum haemorrhage and invasive obstetrical procedures: Bleeding during labor and complicated delivery procedures.
∙ If delivering twins, first twin is at higher risk of infection than second twin: The first twin born in a multiple birth has a higher risk of getting HIV.
∙ Premature baby is at higher risk than term baby: Babies born early are at higher risk.
∙ Mixed feeding carries a higher risk than exclusive breastfeeding or use of replacement feeding: Feeding a baby both breast milk and formula increases the risk compared to feeding only breast milk or only formula.
Investigations
1. ∙ Blood: HIV serological test: Blood tests to detect HIV antibodies or antigens in the mother.
2. ∙ HIV -DNA/ PCR testing of babies: Specialized tests to detect the HIV virus directly in babies.
Management
All HIV services for pregnant mothers are offered in the MCH clinic. After delivery, mother and baby will remain in the MCH postnatal clinic till HIV status of the child is confirmed, then they will be transferred to the general ART clinic: Pregnant women receive all their HIV-related care at the Maternal and Child Health clinic. After the baby is born, both mother and baby stay in the MCH postnatal clinic until the baby’s HIV status is confirmed, and then they are moved to the regular ART clinic if needed.
The current policy aims at elimination of Mother-to-Child Transmission (eMTCT) through provision of a continuum of care with the following elements:
∙ Primary HIV prevention for men, women and adolescents
∙ Prevention of unintended pregnancies among women living with HIV
∙ Prevention of HIV transmission from women living with HIV to their infants
∙ Provision of treatment, care and support to ALL women infected with HIV, their children and their families
Management of HIV Positive Pregnant Mother
Key Interventions for eMTCT ;
∙ Routine HIV Counseling and Testing during ANC (at 1st contact. If negative, repeat HIV test in the third trimester/ labour: All pregnant women should be offered HIV testing and counseling at their first antenatal care visit. If the initial test is negative, the test should be repeated later in pregnancy or during labor.
∙ Enrolment in HIV care if mother is positive and not yet on treatment: If a pregnant woman tests positive for HIV and is not already on medication, she should be enrolled in HIV care.
∙ If mother already on ART, perform viral load and continue current regimen: If the pregnant woman is already taking HIV medication, her viral load (amount of virus in her blood) should be checked, and she should continue her current treatment.
∙ ART in pregnancy, labour and post-partum, and for life – Option B+: The recommended approach is for all pregnant women with HIV to start and continue antiretroviral therapy (ART) for life, starting as early as possible in pregnancy (Option B+).
Treatment
Recommended ARV for option B+
One daily Fixed Dose Combination (FDC) pill containing TDF + 3TC + EFV started early in pregnancy irrespective of the CD4 cell count and continue during labour and delivery, and for life: The preferred medication is a single pill taken daily containing tenofovir disoproxil fumarate (TDF), lamivudine (3TC), and efavirenz (EFV), started as soon as possible in pregnancy, regardless of the woman’s CD4 count, and continued throughout pregnancy, childbirth, and for the rest of her life.
Alternative regimen for women who may not tolerate the recommended option are: ∙
If TDF contraindicated: ABC+3TC+EFV: If there’s a reason why a woman can’t take TDF, an alternative is abacavir (ABC) + 3TC + EFV.
If EFV contraindicated: TDF + 3TC + ATV/r: If EFV is not suitable, another option is TDF + 3TC + atazanavir boosted with ritonavir (ATV/r).
Prophylaxis for opportunistic infections
Cotrimoxazole 960 mg 1 tab daily during pregnancy and postpartum: Pregnant women with HIV should take a daily tablet of cotrimoxazole to prevent certain infections.
NB. Mothers on cotrimoxazole DO NOT NEED IPTp with SP for malaria: Pregnant women taking cotrimoxazole do not need to take sulfadoxine-pyrimethamine (SP) for malaria prevention.
Notes
∙ TDF and EFV are safe to use in pregnancy: The medications TDF and EFV are considered safe for use during pregnancy.
∙ Those newly diagnosed during labour will begin HAART for life after delivery: Women who are diagnosed with HIV for the first time during labor will start lifelong antiretroviral therapy after giving birth.
Caution
∙ In case of low body weight, high creatinine, diabetes, hypertension, chronic renal disease, and concomitant nephrotoxic medications: perform renal function investigations before starting TDF ∙ TDF is contraindicated in advanced chronic renal disease: If a woman has low body weight, kidney problems, diabetes, high blood pressure, or is taking other medications that can harm the kidneys, her kidney function should be checked before starting TDF. TDF should not be used in women with severe chronic kidney disease
We are a supportive platform dedicated to empowering student nurses and midwives through quality educational resources, career guidance, and a vibrant community. Join us to connect, learn, and grow in your healthcare journey
Quick Links
Our Courses
Legal / Policies
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved | Design & Developed by Opensigma

