Oncological Drugs
Subtopic:
Antineoplastic agents
Antineoplastic agents are a category of pharmaceuticals used in the treatment of cancer. These medications work by impeding the development and multiplication of neoplastic cells, which are cancerous cells. They are also commonly known as anticancer drugs.
The therapeutic action of antineoplastic drugs generally operates through two key pathways: influencing the survival mechanisms of cancer cells and bolstering the immune system’s capacity to target and eliminate abnormal cells.
Common Terminology
Alopecia: Refers to hair loss. This is a frequent side effect because these drugs affect all rapidly dividing cells in the body, including hair follicle cells.
Angiogenesis: Describes the process of new blood vessel formation. Cancerous growths stimulate angiogenesis to secure a supply of nutrients necessary for their expansion.
Carcinoma: A type of malignancy originating in epithelial tissues, which line organs and cavities.
Metastasis: The process where cancer cells disseminate from their primary location and establish secondary tumors in other areas of the body.
Neoplasm: Indicates an abnormal tissue mass. Neoplasms can be non-cancerous (benign) or cancerous (malignant).
Sarcoma: A type of malignancy arising from mesenchymal tissues, which include supportive and connective tissues like bone, muscle, and fat.
Anaplasia: Characterized by a loss of cellular differentiation and organizational structure, a hallmark trait of cancerous cells.
Cancer: A broad term denoting a malignant neoplasm or a new, uncontrolled growth of abnormal cells.
Cancer Classification
Cancer can be broadly categorized into two major types:
Solid Tumors: These are physically discernible masses of cancerous tissue. Solid tumors can be further sub-classified by their tissue of origin:
Carcinomas: Develop from epithelial cells, which form the linings of organs and body cavities.
Sarcomas: Originate in mesenchymal cells, the cells of embryonic connective tissue, which give rise to bone, cartilage, muscle, fat, and blood vessels.
Hematological Malignancies: These cancers affect the blood-forming tissues and lymphatic system. They disrupt the body’s ability to produce and regulate blood cells properly and include cancers of the:
Bone marrow (e.g., leukemia).
Lymphatic system (e.g., lymphoma).
Classification of Antineoplastic Agents:
Antineoplastic agents are diverse and classified based on their mechanisms of action against cancer cells.
Alkylating Agents:
These agents function by disrupting DNA replication.
They achieve this by adding alkyl groups to DNA bases, causing DNA damage and preventing proper cell division.
They are generally more effective against cancers that grow slowly because their mechanism primarily targets DNA replication processes that are ongoing during cell division.
Antimetabolites:
These substances are designed to mimic naturally occurring compounds essential for cell function.
They interfere with the synthesis of DNA and RNA by substituting themselves for normal building blocks or inhibiting key enzymes required for nucleic acid production.
This disruption ultimately halts cell growth and division.
Antineoplastic Antibiotics:
These are not antibiotics in the traditional sense of fighting bacterial infections, but rather substances derived from microbial sources that have anticancer properties.
They work by binding directly to DNA, which leads to blockage of RNA synthesis, a process necessary for protein production and cell survival.
They tend to be more cytotoxic to rapidly proliferating cells, making them effective against cancers with high growth rates.
Mitotic Inhibitors:
These agents specifically target the process of cell division (mitosis).
They function by interfering with the formation or function of microtubules, which are critical structures needed for chromosome separation during mitosis.
By disrupting mitosis, they prevent cancer cells from replicating, leading to cell death.
Hormones and Hormone Modulators:
This class includes agents that manipulate hormone levels or hormone action in the body.
They can either block the effects of hormones that stimulate the growth of hormone-sensitive tumors, or in some cases, mimic hormones to alter the cellular environment in a way that is unfavorable for tumor growth.
These are primarily used in cancers like breast and prostate cancer which are known to be hormone-dependent.
Cancer Cell-Specific Agents (Targeted Therapies):
These represent a more modern and precise approach to cancer treatment.
They are designed to selectively target specific molecules within cancer cells that are crucial for their growth, survival, and spread.
By targeting these specific pathways, they aim to minimize damage to healthy, normal cells, potentially reducing systemic toxicity commonly seen with traditional chemotherapy.
Miscellaneous Antineoplastics:
This category encompasses a variety of anticancer drugs that do not neatly fit into the other classifications.
They exhibit diverse mechanisms of action, often unique to each drug within this group.
These agents are frequently considered when standard treatments have proven ineffective or when the specific cancer type responds better to these unique mechanisms.

Cell Cycle Phases
The cell cycle is a series of stages a cell progresses through, leading to division and duplication.
G1 Phase: Growth Phase 1
This initial phase marks a period of significant cell growth. The cell increases in size and actively synthesizes proteins and organelles needed for subsequent phases.
Crucially, during G1, the cell produces and releases enzymes that are essential for the upcoming process of DNA replication.
It is important to note that cells can exit the active cell cycle from G1 and enter a quiescent state known as G0 phase. In G0, cells are not actively dividing; they may be in a state of dormancy or performing their specialized functions without progressing further through the cycle.
S Phase: Synthesis Phase
Often termed the synthetic phase, S phase is characterized by DNA replication.
During this critical stage, the cell meticulously duplicates its entire genome. This involves creating identical copies of each chromosome, ensuring that each daughter cell will receive a complete and accurate set of genetic information.
G2 Phase: Growth Phase 2 & Checkpoint
G2 phase serves as a vital checkpoint phase in the cell cycle.
Following successful DNA replication in S phase, the cell continues to grow and synthesize proteins necessary for cell division.
The checkpoint function of G2 is critical; it ensures that DNA replication has been completed accurately and that the cell is ready for mitosis. Only upon passing this checkpoint will the cell proceed to the next phase, mitosis.
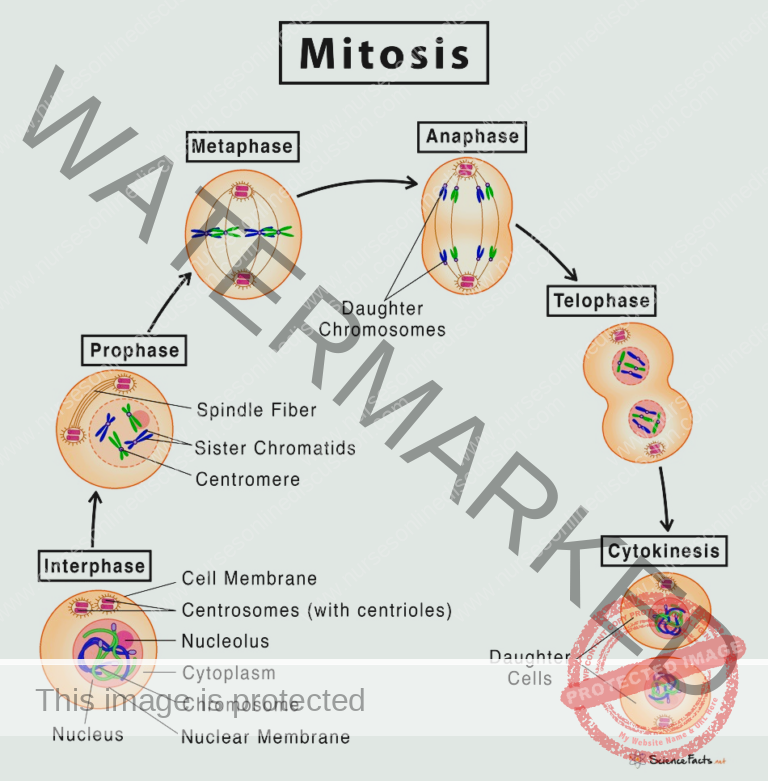
Mitosis Phase: Cell Division
Mitosis is the phase of active cell division, where the cell physically separates into two daughter cells. Mitosis is further subdivided into distinct stages:
Prophase: In prophase, the chromosomes condense, becoming visibly distinct and compact. Simultaneously, the nuclear envelope, which encloses the nucleus, begins to break down, preparing for chromosome segregation.
Metaphase: During metaphase, the microtubules, which are part of the cell’s cytoskeleton, play a crucial role. They extend from structures called centrosomes and attach to the centromeres of each chromosome. These microtubules then orchestrate the alignment of all chromosomes at the cell’s equator or metaphase plate, ensuring equal distribution to daughter cells.
Anaphase: Anaphase is the stage of chromosome separation. The sister chromatids, which are identical copies of each chromosome, are pulled apart by the microtubules towards opposite poles of the cell. This separation ensures that each pole receives a complete set of chromosomes.
Telophase: Telophase is the final stage of nuclear division. Here, the separated chromosomes arrive at opposite poles, and new nuclear envelopes form around each set of chromosomes, creating two distinct nuclei within a single cell.
Cytokinesis: Following mitosis and nuclear division, cytokinesis is the process of cytoplasmic division. This is where the cell physically divides into two separate daughter cells. After cytokinesis, each daughter cell has two potential fates: they may become dormant and enter the G0 phase (Gap 0), or, if conditions are right and signals are received, they may re-enter the cell cycle by proceeding to the G1 phase, starting the process anew and potentially undergoing another round of cell division.
Types of Antineoplastic Drugs
Alkylating Agents/DNA Replication Inhibitors
Alkylating agents function by attaching alkyl groups to DNA. This process hinders the DNA strands’ ability to unwind and separate, which is essential for replication. This mechanism is particularly effective against cancers that are slow-growing as these cells are more reliant on continuous DNA replication for their propagation.
Indications:
Malignancies of the lymphatic system, such as Lymphomas and Hodgkin’s disease
Leukemias (various types)
Myelomas (e.g., multiple myeloma)
Cancers of the reproductive organs including Ovarian, Testicular, and Breast cancers
Pancreatic cancer
Pulmonary carcinoma (lung cancer)
Additionally, used in non-cancerous conditions like Rheumatoid arthritis
Contraindications:
Pregnancy and Breastfeeding: Due to the potential for severe harm to the developing fetus and nursing infant.
Pre-existing Bone marrow suppression: As these agents further depress bone marrow function.
Impaired Renal and Hepatic function: Which can affect drug metabolism and excretion, increasing toxicity.
Adverse Effects:
Gastrointestinal (GI): Manifestations include Nausea, Vomiting, Diarrhea, and damage to the Mucous membranes.
Genitourinary (GU): Potential for Renal toxicity and elevated Uric acid levels in the body.
Hematological: Significant Bone marrow suppression, leading to decreased production of blood cells resulting in Anemia, Thrombocytopenia (low platelets), and Leukopenia (low white blood cells).
Alopecia (Hair loss).
Examples of Alkylating Agents:
Drug Name Indications Dosage Cyclophosphamide (Cytoxan, Neosar) Lymphomas, Leukemias, Myelomas, Breast cancer Induction: 40–50 mg/kg daily IV over 2–5 days; Maintenance: 1–5 mg/kg daily Orally/IV Busulfan (Busulfex, Myleran) Chronic myelogenous leukemia (CML), Lymphomas Induction: 4–8 mg/day Orally; Maintenance: 1–3 mg/day Orally Chlorambucil (Leukeran) Hodgkin’s disease, Non-Hodgkin’s lymphoma 0.1–0.2 mg/kg daily Orally for 3–6 weeks; Maintenance: 0.03–0.1 mg/kg daily Orally
Antimetabolites
Antimetabolites are designed to resemble natural substances crucial for cellular processes. By mimicking these substances, they disrupt the normal pathways of DNA and RNA synthesis. These drugs are most effective against cancer cells that are actively and rapidly dividing.
Indications:
Various types of Leukemias
Cancers of the Gastrointestinal tract
Specific solid tumors such as Breast, Stomach, Pancreas, and Colon cancers
Contraindications:
Pregnancy and Breastfeeding: Due to the risk of developmental abnormalities and harm to the infant.
Pre-existing Bone marrow suppression: As these drugs further impair bone marrow function.
Impaired Renal and Hepatic function: Which can affect drug processing and elimination.
Gastrointestinal ulceration: As antimetabolites can exacerbate existing ulcers.
Adverse Effects:
Central Nervous System (CNS): May cause Headache, Drowsiness, and Dizziness.
Respiratory: Potential for Pulmonary toxicity, including Interstitial pneumonitis.
Hematological: Bone marrow suppression, leading to blood cell deficiencies.
Gastrointestinal (GI): Symptoms include Nausea, Vomiting, Diarrhea, and potential Hepatic toxicity (liver damage).
Genitourinary (GU): Risk of Renal toxicity.
Examples of Antimetabolites:
Drug Name Indications Dosage Methotrexate (Rheumatrex, Trexall) Leukemias, Rheumatoid arthritis 15–30 mg Orally/IM depending on the specific condition being treated Fluorouracil (Adrucil, Carac) Breast, stomach, colon cancer 12 mg/kg daily IV on days 1–4, then 6 mg/kg IV on days 6, 8, 10, and 12
Antineoplastic Antibiotics
Antineoplastic antibiotics are a class of drugs that, unlike traditional antibiotics for bacterial infections, are used for their anticancer properties. They function by directly interacting with DNA, which subsequently inhibits RNA synthesis. This action is particularly effective against cells that are rapidly dividing, a characteristic of many cancer types.
Indications:
Testicular cancer
Lymphomas
Squamous cell carcinoma
Choriocarcinoma
Contraindications:
Pregnancy and Breastfeeding: Due to risks of fetal harm and infant exposure.
Pre-existing Bone marrow suppression: As these drugs further reduce bone marrow activity.
Impaired Renal and Hepatic function: Which can affect drug clearance and increase toxicity.
Pre-existing Pulmonary or Cardiac conditions: As these drugs can exacerbate these conditions.
Adverse Effects:
Central Nervous System (CNS): May induce Headache, Drowsiness, and Dizziness.
Respiratory: Risk of Pulmonary toxicity.
Hematological: Bone marrow suppression, leading to decreased blood cell production.
Gastrointestinal (GI): Includes Nausea, Vomiting, and potential Hepatic toxicity.
Genitourinary (GU): Risk of Renal toxicity.
Alopecia (Hair loss).
Examples of Antineoplastic Antibiotics:
Drug Name Indications Dosage Bleomycin (Blenoxane) Testicular cancer, Lymphoma Test dose of 1-2 units given 2-4 hours before therapy; 0.25–0.5 units/kg IM, IV, or SC once/twice weekly Doxorubicin (Adriamycin, Doxil) Breast cancer, Kaposi’s sarcoma 60–75 mg/m² as a single IV dose; repeat every 21 days
Mitotic Inhibitors/Vinca Alkaloids
Mitotic inhibitors, including Vinca alkaloids, work by disrupting cell division. They specifically target mitosis, particularly the M phase of the cell cycle, by interfering with the formation and function of microtubules, which are essential for chromosome separation during cell division.
Indications:
Leukemia
Lymphomas, including Hodgkin’s lymphoma
Kaposi’s sarcoma
Testicular and Breast cancer
Contraindications:
Pregnancy and Breastfeeding: Because of the potential for developmental and infant harm.
Pre-existing Bone marrow suppression: As these agents further impair bone marrow function.
Impaired Renal and Hepatic function: Which can affect drug metabolism and elimination.
Gastrointestinal ulceration: As these drugs can worsen existing ulcers.
Adverse Effects:
Central Nervous System (CNS): May cause Headache, Drowsiness, and Dizziness.
Hematological: Bone marrow suppression, resulting in reduced blood cell counts.
Gastrointestinal (GI): Nausea, Vomiting, and Mucous membrane deterioration.
Genitourinary (GU): Risk of Renal toxicity.
Alopecia (Hair loss).
Neuropathy (nerve damage), Stomatitis (inflammation of the mouth), Constipation.
Examples of Mitotic Inhibitors:
Drug Name Indications Dosage Vincristine (Oncovin, Vincasar) Leukemia, Lymphoma Adult: 1.4 mg/m² IV at weekly intervals Vinblastine (Velban) Hodgkin’s disease, Lymphoma Adult: 3.7 mg/m² IV once weekly; Pediatric: 2.5 mg/m² IV once weekly
Hormones and Hormone Modulators
Certain cancers, especially those in tissues like the breast, ovaries, uterus, prostate, and testes, are sensitive to hormonal influences, particularly estrogen. In these hormone-sensitive cancers, estrogen can bind to estrogen-receptor sites on tumor cells, stimulating their growth and division. Hormones and hormone modulators are used to counteract this by blocking or interfering with these receptor sites, thus inhibiting cancer growth and promoting cell death. Some agents also reduce the production of gonadotropic hormones if the tumors are responsive to these hormones, while others directly block androgen receptors.
Indications:
Breast cancer in postmenopausal women
Prostate cancer
Contraindications and Cautions:
Known allergy to the drug: To prevent Hypersensitivity reactions.
Hypercalcemia: Contraindicated with toremifene because it can elevate serum calcium levels.
Pregnancy and Breastfeeding: Due to potential severe harm to the fetus and neonate.
Bone marrow suppression: To guide re-dosing and determine appropriate dose levels.
Impaired Renal and Hepatic function: As these conditions can interfere with drug metabolism and excretion.
Known GI ulceration or ulcerative diseases: Which can be exacerbated by these medications.
Adverse Effects:
Menopausal symptoms: Including Hot flashes, Vaginal dryness, and Mood changes.
Hematological: Bone marrow suppression.
Gastrointestinal (GI): Hepatic toxicity.
Genitourinary (GU): Renal toxicity.
Hypercalcemia (elevated calcium levels in the blood).
Examples of Hormones and Hormone Modulators:
Drug Name Indications Dosage Tamoxifen (Nolvadex) Breast cancer 20–40 mg Orally per day Anastrozole (Arimidex) Breast cancer in postmenopausal women 1 mg Orally per day
Cancer Cell-Specific Agents (Targeted Therapies)
Cancer cell-specific agents represent a targeted approach to cancer treatment. Unlike traditional chemotherapy, these drugs are designed to selectively act on specific molecules or pathways critical to cancer cell growth and survival, aiming to minimize damage to healthy cells and enhance efficacy against particular cancer types. Three main groups fall under this category: protein tyrosine kinase inhibitors, epidermal growth factor inhibitors, and proteasome inhibitors.
Therapeutic Action:
Protein Tyrosine Kinase Inhibitors: These agents target specific enzymes known as protein tyrosine kinases, which are crucial for protein synthesis and cell signaling in certain tumor cells. By blocking these enzymes, they inhibit tumor cell growth and division. A key advantage is their relative specificity, leading to fewer adverse effects compared to traditional chemotherapy as they less dramatically affect healthy human cells. Examples include everolimus (Afinitor), gefitinib (Iressa), imatinib (Gleevec), lapatinib (Tykerb), nilotinib (Tasigna), sorafenib (Nexavar), sunitinib (Sutent), and temsirolimus (Torisel).
Epidermal Growth Factor Inhibitors: These drugs target epidermal growth factor receptors (EGFRs), which are proteins involved in cell growth and are often overexpressed in cancer cells. While EGFRs are present in both normal and cancerous cells, they are more abundant in rapidly growing cancer cells. Erlotinib (Tarceva) is an example.
Proteasome Inhibitors: These agents inhibit proteasomes, large protein complexes in cells responsible for maintaining cell homeostasis and regulating protein production by degrading unnecessary or damaged proteins. By inhibiting proteasomes, these drugs disrupt cellular balance, leading to cell death, particularly in cancer cells. Bortezomib (Velcade) is a representative drug in this class.
Indications:
Cancer cell-specific agents are indicated for specific cancers based on their molecular targets:
Imatinib: A protein tyrosine kinase inhibitor, is approved for the treatment of chronic myelocytic leukemia (CML). It targets the Bcr-Abl tyrosine kinase, an abnormal enzyme resulting from the Philadelphia chromosome, which is characteristic of CML.
Bortezomib: A proteasome inhibitor, is used in the treatment of multiple myeloma, particularly in cases where the disease has progressed despite standard therapies.
Contraindications and Cautions:
Pregnancy: All drugs in this class are classified as Pregnancy Category D, indicating potential fetal risk.
Women of Childbearing Age: Should use effective barrier contraception during treatment.
Breastfeeding: These drugs may pass into breast milk, and use during lactation should be carefully considered, weighing benefits against potential risks.
Hepatic Dysfunction: Caution is advised, particularly with imatinib, due to an increased risk of toxicity.
Nilotinib: Contraindicated in patients with or at risk for prolonged QT intervals (e.g., those with hypokalemia, hypomagnesemia, or taking other QT-prolonging drugs) due to the risk of QT prolongation and potential for sudden death.
Known Allergy: To prevent hypersensitivity reactions.
Adverse Effects:
Adverse effects vary by agent but generally are less severe than traditional chemotherapy:
Imatinib: May cause GI upset, muscle cramps, heart failure, fluid retention, and skin rashes. Severe adverse effects typical of traditional chemotherapy, such as severe bone marrow depression, alopecia, and severe GI issues, are less common.
Gefitinib: Associated with potentially severe interstitial lung disease and various eye symptoms.
Pazopanib: May lead to some bone marrow depression, diarrhea, hypertension, liver impairment, and hair color changes.
Lapatinib: Can cause diarrhea, liver impairment, and altered heart function.
Erlotinib and Bortezomib: Linked to cardiovascular events and pulmonary toxicity.
Bortezomib: May cause peripheral neuropathy and liver and kidney impairment.
Platinum Analogues/Miscellaneous Antineoplastics
This group includes drugs with diverse mechanisms of action that are not completely understood.
Examples of Miscellaneous Antineoplastics:
Cisplatin: Dosage range 20–70 mg/m² IV
Carboplatin: Dosage 360 mg/m² IV
Hydroxyurea: Dosage 20–80 mg/kg PO (belongs to substituted ureas class)
Indications:
Testicular cancer
Ovarian cancer
Bladder cancer
Hydroxyurea is also used for Sickle cell crisis prevention.
Contraindications, Side Effects: Generally similar to other antineoplastic agents, including bone marrow suppression, GI distress, renal and hepatic toxicity, and alopecia.
Nursing Considerations for Antineoplastic Agents
Nursing Assessment:
Key aspects for nursing assessment when administering antineoplastic agents include:
Assess for Contraindications and Cautions: Evaluate for pre-existing conditions or factors that contraindicate or require caution with antineoplastic drug use, such as drug allergies, hepatic or renal impairment, bone marrow suppression, pregnancy, and lactation, to prevent potential complications.
Comprehensive Physical Assessment: Conduct a thorough physical assessment, including reviewing current medications, assessing orientation and reflexes, vital signs, and bowel sounds. This establishes baseline data before initiating drug therapy, aids in evaluating therapy effectiveness, and helps identify any adverse effects related to drug therapy.
Laboratory Monitoring: Regularly monitor laboratory results, particularly Complete Blood Count (CBC) with differential, to detect potential bone marrow suppression and drug toxicity, and to guide appropriate drug dosing. Liver and renal function tests are also essential to assess organ function, determine the need for dose adjustments, and identify drug-induced toxic effects.
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved Design & Developed by Opensigma.co

