Managing Children Living with HIV/AIDS
Subtopic:
Treatment of HIV/AIDS in Children (ARV Therapy)
Initiating treatment for HIV/AIDS in children follows confirmation of the diagnosis through specific diagnostic procedures.
Diagnostic Measures and Investigations
Criteria for HIV/AIDS Diagnosis:
Clinical Staging: Evaluation based on clinical stage criteria for HIV infection.
Positive HIV Blood Test: A positive result from an HIV blood test is essential for diagnosis.
A reactive rapid HIV test result necessitates confirmation before an HIV infection diagnosis can be officially given. ELISA Ag/Ab combination tests are frequently employed as a screening tool, particularly for blood donations, to identify individuals who may be in the “window period” of infection, where antibodies may not yet be detectable.
PCR (Polymerase Chain Reaction) tests, a type of Nucleic Acid Amplification Testing (NAT), are designed to directly detect the genetic material of HIV itself, rather than relying on antibody or antigen detection.
Important Note: It’s crucial to interpret HIV test results in the context of the individual’s clinical status, medical history, and risk factors for HIV exposure.
HIV Testing Services (HTS) Provision Protocol
The standard protocol for providing HIV testing services involves a series of sequential steps:
Step 1: Pre-Test Information and Counseling
Provide essential information to the client/patient, including:
HIV Transmission Routes: Explain how HIV is transmitted from person to person.
Basic HIV Prevention Measures: Discuss strategies to prevent HIV infection.
Benefits of HIV Testing: Outline the advantages of knowing one’s HIV status.
Possible Test Results: Describe the range of potential test outcomes (positive, negative, indeterminate).
Available Services: Inform about HIV care, treatment, and support services.
Consent and Confidentiality: Emphasize the importance of informed consent and the assurance of confidentiality.
Individual Risk Assessment: Conduct a personalized assessment of the client’s HIV risk factors.
HTS Card Completion: Complete the necessary Health Testing Services (HTS) card for documentation.
Question and Answer Session: Encourage clients/patients to ask questions and address their concerns.
Step 2: HIV Testing
Blood Sample Collection: HIV testing is performed using a blood sample.
Age-Based Testing Methods:
Below 18 Months: DNA PCR test is the standard test for infants and children under 18 months old.
Above 18 Months: Antibody tests are typically used for individuals older than 18 months.
HIV Testing Algorithms: Refer to national HIV testing algorithms for age-specific guidelines and procedures.
Step 3: Post-Test Counseling (Individual/Couple)
Readiness Assessment: Assess the client’s readiness to receive their test results.
Result Delivery: Communicate test results in a clear and understandable manner.
Address Concerns: Provide an opportunity to address any immediate concerns or questions.
Disclosure and Partner Testing: Discuss the importance of HIV status disclosure and encourage partner testing for those who test positive.
Risk Reduction Counseling: Reinforce risk reduction strategies and safe practices.
Information on HIV Care and ART: Provide information about basic HIV care and the availability of Antiretroviral Therapy (ART).
HTS Card and Register Completion: Ensure accurate completion of the HTS card and HTS register for record-keeping.
Step 4: Linkage to Other Services
Service Information: Provide detailed information about relevant HIV prevention, treatment, care, and support services available.
Referral Form: Complete a triplicate referral form to facilitate linkage to services.
Enrollment and Register Updates: Upon patient enrollment in pre-ART or ART services, record the pre-ART enrollment number in the HTS register and subsequently update the ART register once ART is initiated.
Principles of HIV Testing Services (HTS)
The delivery of HTS must adhere to ethical and rights-based principles, ensuring services are:
Non-Discriminatory: Offered equitably to all individuals without prejudice or bias.
Human Rights Approach: Respectful of human rights and dignity.
Guided by the 5Cs Principles:
Confidentiality:
Providers must ensure privacy throughout the HTS process.
Client information shared during HTS must not be disclosed to any other person without the client’s explicit consent.
Consent:
Age of Consent: Individuals aged 12 years and above are considered competent to provide consent for HTS on their own behalf.
Proxy Consent: In situations where individual consent cannot be obtained (e.g., for younger children or individuals lacking capacity), consent should be obtained from a parent or legal guardian (for a child), next of kin, or a legally authorized representative.
Counseling:
Quality Counseling: All individuals accessing HTS must receive high-quality counseling both before and after HIV testing, adhering to the approved national HTS protocol.
Correct Test Result:
National Testing Algorithm Adherence: HTS providers are obligated to strictly follow the national HIV testing algorithm.
Standard Operating Procedures (SOPs): Providers must adhere to Standard Operating Procedures for HIV testing to guarantee clients receive accurate and reliable HIV test results.
Connection to Appropriate Services:
Linkage to Care: Providers are responsible for linking HTS clients to appropriate and necessary HIV prevention, treatment, care, and support services based on their individual needs and test results.
Recommended Sites for Blood Sample Collection in Children
When performing blood collection for HIV testing in children, appropriate site selection depends on age and size:
Infants (1-4 months, <6kg): Heel prick is generally the most suitable and effective site.
Infants (5-10 months, <10kg): Toe prick is often preferred for sample collection in this age group.
Larger Infants and Older Children: Use the ring finger or middle finger for finger prick blood collection.
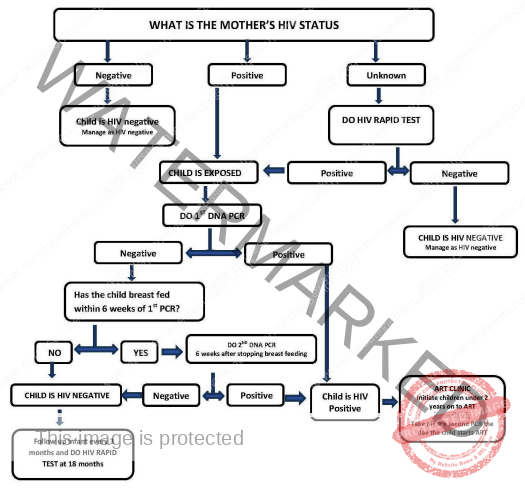
HIV Testing Algorithm for Infants and Children Under 18 Months
For infants and children below 18 months of age, a virological test, specifically DNA PCR, is the recommended method for determining HIV status. Samples for testing are typically collected using dried blood spot (DBS) specimens. The timing and interpretation of test results are guided by the following algorithm:
Initial DNA PCR Test:
Timing: The first DNA PCR test should be performed at six weeks of age or as soon as possible after that age.
Positive Result:
HIV Infection Confirmed: A positive DNA PCR test result definitively indicates that the child is HIV-infected.
ART Initiation: All infants with a positive DNA PCR test result should be started on Antiretroviral Therapy (ART) immediately.
Confirmatory Sample: A second blood sample should be collected on the same day ART is initiated to confirm the initial positive DNA PCR test result.
Negative Result:
No Current Infection, but Potential Risk: A negative first DNA PCR test result indicates that the child is not currently infected with HIV. However, if the infant is still breastfeeding from a mother living with HIV, there remains a risk of HIV transmission through breast milk.
Repeat DNA PCR Testing: Infants with a negative initial DNA PCR test result should be retested using DNA PCR at six weeks after breastfeeding cessation.
Final Antibody Test at 18 Months: For infants with a second negative DNA PCR test, a final rapid antibody test should be performed at 18 months of age to definitively exclude HIV infection, ensuring any passively transferred maternal antibodies have waned.
Use of Antibody and PCR Tests in Children
Antibody Tests in Children <18 Months: Antibody tests have specific applications in infants under 18 months:
Determine HIV Exposure: To identify HIV exposure in infants born to mothers whose HIV status is unknown at delivery.
Exclude Infection at 18 Months: To rule out HIV infection at 18 months of age in children who have ceased breastfeeding for at least 6 weeks, confirming the absence of infection once maternal antibodies have disappeared.
Antibody Tests in Children >18 Months: To confirm HIV infection in children older than 18 months.
HIV DNA PCR Testing: HIV DNA PCR testing is essential in specific situations:
All HIV-Exposed Babies: Every infant born to a mother living with HIV should undergo HIV DNA PCR testing at 6 weeks of life or at their first clinic visit if older than 6 weeks.
Figure showing HIV testing algorithm for children <18 months of age
Procedure for Dried Blood Spot (DBS) Collection
The process for collecting a Dried Blood Spot (DBS) sample involves a series of steps to ensure sample quality and minimize discomfort for the infant or child:
Warm the Collection Site: Gentle warming of the intended puncture area is crucial. This can be achieved by:
Applying a warm compress briefly to the heel or finger.
Warming enhances blood flow to the area, making sample collection easier.
Position the Infant: Proper positioning of the infant is essential for safety and ease of access to the heel:
Hold the baby securely with the foot positioned downwards. Gravity assists blood flow to the puncture site.
Sterilize the Puncture Site: Strict aseptic technique is vital to prevent infection:
Cleanse the chosen puncture area (heel or finger) thoroughly with an alcohol swab.
Allow Alcohol to Air Dry: It is critical to let the alcohol air dry completely before proceeding with the puncture.
Residual alcohol can dilute the blood sample, potentially affecting test results, and may also cause stinging sensation to the infant.
Skin Puncture: Perform the skin prick using a sterile lancet:
Firmly press the lancet against the prepared site on the foot (heel) or finger.
Prick the skin with a swift, decisive motion to obtain adequate blood flow.
Wipe Away First Drop of Blood: Remove the initial drop of blood that appears at the puncture site.
The first drop may be diluted with tissue fluid and is not ideal for accurate DBS testing. Use a clean, dry gauze pad to gently wipe it away.
Collect a Large Blood Drop: Encourage the formation of a substantial blood drop at the puncture site.
Allow a large, hanging drop of blood to naturally collect. Avoid excessive squeezing or milking of the site, which can dilute the sample with tissue fluid.
Apply Blood to DBS Card Circles: Transfer the blood drop to the DBS card:
Touch the filter paper circle on the DBS card to the blood drop.
Allow approximately 50 microliters (µl) of blood, roughly equivalent to two drops, to soak into one circle on the DBS card.
Ensure the blood completely fills the circle and saturates the filter paper to the edges.
Fill Multiple Circles: Collect sufficient blood volume by filling multiple circles on the DBS card.
Fill at least three circles on the DBS card with blood to provide an adequate sample volume for testing and potential retesting if needed.
Clean Puncture Site: After sufficient blood collection, provide post-puncture care:
Clean the puncture site with a clean gauze pad.
Do not apply a bandage to the puncture site, as this can trap moisture and potentially lead to skin irritation or infection in infants.
Proper Disposal: Dispose of all materials used in the DBS collection process in accordance with safety protocols.
Dispose of all contaminated materials, including lancets, gauze, and alcohol swabs, into an appropriate sharps container and biohazard waste receptacle.
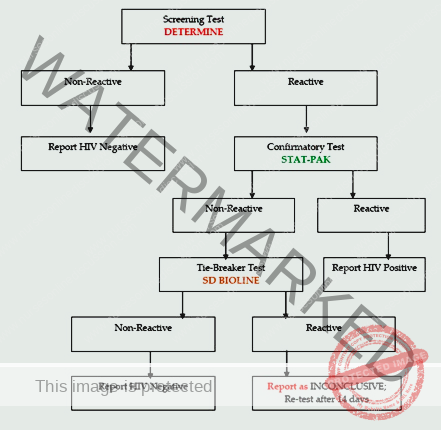
Figure showing serial HIV testing algorithm for persons above 18 months of age
Cautions During HIV Testing
To ensure the accuracy and reliability of HIV test results, it is essential to adhere to the following precautions during testing procedures:
Avoid Expired Test Kits: Never use HIV test kits that have passed their expiration date. Expired kits may yield inaccurate or invalid results.
Do Not Modify Procedures: Strictly avoid any modifications to the recommended testing procedures. Deviations from the validated protocol can compromise test performance.
Use Proper Sample Type: Do not use clotted blood for HIV testing, as clots can interfere with test reagents and result interpretation.
Ensure Sample Integrity: Prevent contamination of blood samples. Avoid using “dirty” blood samples that contain:
Skin flakes.
Powder (e.g., from gloves).
Sweat.
Contaminants can lead to inaccurate results or test failure.
Minimize Air Bubbles: When adding the blood sample to the testing device, carefully avoid introducing air bubbles into the device wells or reaction areas. Air bubbles can disrupt reagent flow and test reactions.
Adhere to Volume and Timing Instructions: Do not deviate from specified volumes or incubation times:
Do Not Alter Blood Volume: Do not add more or less blood than the volume specified in the test kit instructions.
Do Not Alter Buffer Volume: Do not add more or less buffer solution than the recommended amount.
Do Not Exchange Buffers: Do not substitute or exchange buffers between different test kits or lots. Use only the buffer provided with the specific test kit.
Prevent Buffer Contamination: Avoid contaminating the buffer solution. Use aseptic technique when handling buffers.
Do Not Modify Incubation Time: Do not change the recommended incubation time. Adhere strictly to the specified incubation period for accurate results.
LINKAGE FROM HIV TESTING TO HIV PREVENTION, CARE, AND TREATMENT
Linkage in the context of HIV services refers to the crucial process of connecting individuals who have tested positive for HIV from one service point to another. This ensures they access the necessary follow-up services for care and treatment.
Successful Linkage is defined as when a patient/client actually receives the services to which they have been referred. For all individuals who test HIV-positive, timely linkage is essential:
Same-Facility Linkage: Linkage should ideally occur within seven days when referral is within the same healthcare facility.
Inter-Facility or Community-to-Facility Linkage: For referrals between different facilities or from community testing sites to facilities, linkage should be completed within 30 days.
Lay Providers as Linkage Facilitators: It is recommended to utilize lay providers, including community-based and facility-based workers, as linkage facilitators. These individuals can effectively support patients in navigating the linkage process.
Process of Internal Linkage Facilitation (Within the Same Health Facility)
Post-Test Counseling (Following a Positive HIV Test Result)
Accurate Result Delivery: Provide the HIV-positive test result to the patient clearly and sensitively.
Information on Available Care:
Inform the patient about the HIV care and treatment services available at the current facility.
Provide details about services available elsewhere in the facility’s catchment area or referral network.
Describe Next Steps: Clearly outline the immediate next steps in accessing care and treatment.
Benefits of Early Treatment:
Discuss the advantages of initiating treatment early in HIV infection.
Explain the potential disadvantages of delaying treatment initiation.
Barrier Identification and Resolution:
Identify any potential barriers that may hinder the patient’s linkage to care (e.g., transportation, stigma, childcare).
Address and work to resolve these barriers to facilitate access to services.
Shared Decision-Making:
Involve the parent and child (if a child is the patient) in the decision-making process regarding their HIV care and treatment plan.
Documentation:
Client Card: Complete the client card with relevant information.
Referral Notes: Include detailed referral notes for the receiving clinic.
Triplicate Referral Form: Fill out the triplicate referral form for formal referral documentation.
Linkage Facilitator Introduction:
Introduce and hand over the patient to a designated linkage facilitator who will guide them through the internal linkage process.
Appointment Scheduling and Follow-Up:
Book Appointment: If same-day linkage to the HIV clinic is not immediately possible, schedule a specific appointment for the client at the HIV clinic.
Follow-Up: Implement a system to actively follow up with the patient to ensure they attend their scheduled appointment and successfully link to care.
Patient Escort to HIV Clinic
Linkage Facilitator Escort: The linkage facilitator personally escorts the client to the designated ART (Antiretroviral Therapy) clinic within the facility.
Linkage Form Handover: The facilitator hands over the client and all relevant linkage forms to the responsible staff at the ART clinic.
Enrollment at HIV Clinic
Pre-ART Register Enrollment: ART clinic staff register the patient in the pre-ART (pre-Antiretroviral Therapy) register.
Medical Record Creation: Open an HIV/ART card/file to establish a medical record for the patient at the clinic.
ART Preparatory Counseling: Provide ART preparatory counseling to educate the patient about ART, adherence, and expectations.
Baseline Investigations: Conduct necessary baseline laboratory investigations as per protocol before ART initiation.
ART Initiation (if Ready):
Assess Readiness: Evaluate the patient’s readiness to start ART.
Initiate ART: If the patient is deemed ready and willing, initiate Antiretroviral Therapy (ART) according to national guidelines.
Ongoing Counseling Support: Continue to provide ongoing counseling support, addressing key areas such as:
Disclosure: Support with HIV status disclosure to family or partners.
Psychosocial Issues: Address any psychosocial challenges or needs.
Integrated Care Coordination: Coordinate integrated care with other relevant services if needed, such as:
TB/HIV Treatment Integration: For patients co-infected with Tuberculosis (TB).
PMTCT Integration: For women in Prevention of Mother-to-Child Transmission (PMTCT) programs.
Appointment Scheduling: Discuss and schedule an appropriate follow-up appointment with the patient to ensure continuity of care.
Inter-Facility Linkage (Referral to Another Facility)
Inter-facility linkage involves connecting a newly diagnosed patient from one healthcare facility to a different facility for HIV treatment, care, and support services. This is necessary when services are not available at the initial testing site or when patient preference dictates referral.
Referral Tracking and Follow-Up: The referring facility bears the responsibility to actively track and follow up with all HIV-positive patients who are referred to other facilities.
Enrollment and ART Initiation Confirmation: The referring facility must ensure that referred patients are:
Enrolled in care at the receiving facility.
Initiated on ART within a maximum of 30 days from the date of referral.
Follow-Up/Tracking Schedule: Implement a structured follow-up and tracking schedule to monitor referral outcomes and patient linkage to care at the receiving facility.
Community-Facility-Community Linkages
Community-facility linkage is crucial for connecting individuals who test HIV-positive in community-based testing settings (e.g., outreach events, mobile clinics) to formal health facilities for ongoing HIV treatment, care, and support.
Functional Community Health Systems: HTS programs should establish robust community health systems that incorporate effective linkage systems.
Community Linkage Facilitators: Utilize community-based resources and personnel as linkage facilitators, including:
Peer Leaders: Individuals living with HIV who provide peer support and navigation.
Expert Clients: Experienced clients in HIV care who can mentor and support newly diagnosed individuals.
Village Health Teams (VHTs): Community health volunteers.
Community Health Extension Workers (CHEWs): Frontline health workers based in communities.
Community Mobilization and Follow-Up: These community-based linkage facilitators should be actively involved in:
Mobilization for Targeted Outreaches: Engaging communities and individuals for targeted HIV testing outreach activities.
Follow-Up for Linkage: Conducting proactive follow-up to link all individuals who test positive in the community to health facilities.
Timely Community-to-Facility Linkage: Linkage from community testing sites to health facilities should be completed within 30 days after HIV diagnosis to ensure prompt access to care and treatment.
10-Point Care Package for Comprehensive Pediatric AIDS Care
A comprehensive approach to pediatric AIDS care encompasses a 10-point care package addressing key aspects of management:
Early HIV Status Confirmation: Confirm HIV status as early as possible in all infants and children at risk or with presumptive signs/symptoms.
Growth and Development Monitoring: Regularly monitor the child’s growth and development to detect and address any delays or issues.
Immunization Schedule: Ensure immunizations are started and completed as per the national immunization schedule to protect against vaccine-preventable diseases.
Opportunistic Infection (OI) Prophylaxis: Provide prophylaxis for opportunistic infections (OIs), such as Pneumocystis pneumonia (PCP) and tuberculosis, to prevent common and serious infections in HIV-infected children.
Active Infection Management: Actively look for and treat infections early and aggressively. Promptly diagnose and manage both opportunistic and common childhood infections.
Counseling and Education for Mother and Family: Provide comprehensive counseling and education to the mother and family on crucial aspects of care, including:
Optimal Infant Feeding Practices: Guidance on appropriate infant feeding methods in the context of HIV, considering breastfeeding and alternatives.
Good Personal and Food Hygiene: Emphasize the importance of hygiene practices to prevent infections.
Follow-Up Recommendations: Ensure understanding of and adherence to follow-up appointments and recommended care plans for the child.
Disease Staging: Conduct disease staging for the HIV-infected child using appropriate clinical and immunological criteria to assess disease progression and guide treatment decisions.
Antiretroviral Therapy (ART): Offer Antiretroviral Therapy (ART) for the HIV-infected child if needed according to national guidelines, considering factors like clinical stage and CD4 count.
Psychosocial Support: Provide psychosocial support for both the infected child and the mother/family. Address emotional, social, and psychological needs related to HIV diagnosis and care.
Referral for Specialized Care: Refer the infected child to higher levels of specialized care if necessary. This includes referral to specialists or centers with expertise in managing complex pediatric HIV cases or specific complications.
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved Design & Developed by Opensigma.co

