Pediatric Conditions of the Respiratory System
Subtopic:
Respiratory Distress Syndrome

Infant respiratory distress syndrome (IRDS), also known as neonatal respiratory distress syndrome, and historically termed hyaline membrane disease (HMD), is a condition affecting premature infants. It arises from the underdevelopment of the lungs, specifically a lack of sufficient pulmonary surfactant production coupled with structural immaturity.
Respiratory distress syndrome (RDS) is a breathing problem seen in babies born prematurely whose lungs have not yet reached full development. The earlier the baby is born, the greater the chance of developing RDS and requiring supplemental oxygen and breathing assistance.
The primary cause of RDS is a deficiency of surfactant in the infant’s lungs. Surfactant, a vital fluid, begins to be produced in the lungs around 26 weeks of gestation. As the baby develops in the womb, the lungs progressively synthesize more surfactant.
Surfactant is a crucial substance that lines the interior of the lungs. Its primary function is to prevent the air sacs (alveoli) from collapsing so that the infant can effectively breathe air after birth.
Causes of Respiratory Distress Syndrome
Lack or insufficient surfactant: The primary cause of RDS is a deficiency or absence of surfactant, a critical substance needed for proper lung function.
Neonatal infection: Infections in the newborn can sometimes lead to or worsen RDS.
Genetic problem with surfactant production: In rare cases, genetic mutations can disrupt the production of surfactant-associated proteins, leading to RDS.
Risk Factors
Premature birth (before 37 weeks): Babies born before their due date are at significantly higher risk due to underdeveloped lungs and insufficient surfactant.
A sibling with respiratory distress syndrome: A family history of RDS increases the likelihood of a newborn developing the condition, suggesting a possible genetic component or shared environmental factors.
Multiple pregnancy (twins, triplets): Multiple births often result in premature delivery, increasing the risk of RDS.
Impaired blood flow to the baby during delivery: Conditions that restrict blood flow to the baby during birth can compromise lung development and surfactant production.
Delivery by cesarean: Babies born via Cesarean section, particularly before labor begins, may have a higher risk as they might not experience the same hormonal changes that prepare the lungs for breathing.
Maternal diabetes: Infants born to mothers with diabetes can have delayed lung maturation and reduced surfactant production due to high insulin levels.
Infection: Maternal infections that are passed to the baby can interfere with lung development and surfactant production.
Induction of labor before the baby is full-term: Electively inducing labor before 39 weeks increases the risk of delivering a baby with immature lungs.
Multiple pregnancy (twins or more): Carrying multiple babies often leads to earlier delivery.
Cold stress: Difficulty maintaining body temperature can worsen respiratory distress in newborns.
Patent ductus arteriosus (PDA): This heart condition, common in premature infants, can worsen lung problems.
Rapid labor: A very quick delivery may not allow the baby’s lungs enough time to clear fluid and adapt to breathing air.
Prematurity: Being born too early is the most significant risk factor for RDS.
Pathophysiology
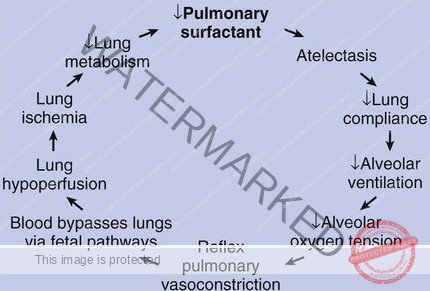
The underlying problem in RDS is that the lungs of affected infants are deficient in surfactant. Surfactant is essential because it reduces surface tension within the alveoli (tiny air sacs in the lungs). This reduced surface tension is what prevents the alveoli from collapsing at the end of each breath, allowing for efficient gas exchange.
This surfactant deficiency can be exacerbated by high levels of insulin in newborns, especially those born to mothers with diabetes. Insulin can interfere with surfactant production.
The lack of sufficient surfactant leads to uneven inflation of the alveoli during inhalation, meaning some alveoli inflate while others don’t. Crucially, it also causes alveoli to collapse at the end of exhalation.
Because the alveoli keep collapsing, the infant has to work very hard with each breath to re-inflate them. This increased effort leads to exhaustion, making it progressively harder for the infant to open the alveoli.
The widespread inability to maintain lung expansion results in atelectasis, which is the collapse of lung tissue. This progressive atelectasis, along with the lack of alveolar stability, causes an increase in pulmonary vascular resistance. Normally, pulmonary vascular resistance decreases after birth to allow for proper blood flow to the lungs.
This increased resistance leads to pulmonary hypertension, meaning high blood pressure within the lung tissue. As a consequence, there is a decrease in effective pulmonary blood flow, hindering the lungs’ ability to oxygenate the blood.
Phases of ARDS (Pathogenesis)
ARDS progresses through three distinct phases: the exudative phase, the proliferative phase, and the fibrotic phase.
1. Exudative Phase
During this initial stage, injury occurs to the endothelial cells lining the alveolar capillaries and the type I pneumocytes, which are the epithelial cells of the alveoli. This damage disrupts the normally tight alveolar barrier, allowing fluid and large molecules to move across. This leakage results in the accumulation of protein-rich fluid in both the interstitial spaces and the alveoli themselves. Simultaneously, there is an increase in pro-inflammatory cytokines during this acute period. These cytokines trigger the recruitment of immune cells, predominantly neutrophils, into the lung tissue and the air spaces of the alveoli. Plasma proteins mix with cellular debris and dysfunctional pulmonary surfactant within the air spaces, forming characteristic hyaline membrane whorls. The build-up of alveolar edema significantly reduces the air-filled volume of the lungs. The collapse of larger areas, particularly in the lower, gravity-dependent parts of the lungs, contributes to a decrease in the overall stretchiness or compliance of the lung tissue. This leads to blood passing through the lungs without being adequately oxygenated (intrapulmonary shunting), resulting in hypoxemia. Consequently, the effort required to breathe increases, causing dyspnea (shortness of breath). The exudative phase typically spans the first week (7 days) following exposure to a factor that triggers ARDS. The increased respiratory rate (tachypnea) and the heightened effort of breathing frequently lead to respiratory muscle fatigue and eventually respiratory failure.
2. Proliferative Phase
This phase of ARDS generally extends from day 7 to day 21. A significant number of patients show rapid improvement and are able to be taken off mechanical ventilation during this period. However, despite this progress, many individuals still experience symptoms such as dyspnea, tachypnea, and hypoxemia. Microscopic examination of lung tissue often reveals the first signs of recovery during this phase. The lung begins to repair itself, the accumulated material in the alveoli starts to organize, and there is a change in the type of immune cells present, shifting from predominantly neutrophils to lymphocytes in the pulmonary infiltrates. As a key part of the repair process, type II pneumocytes multiply along the alveolar basement membranes. These specialized epithelial cells are crucial as they produce new pulmonary surfactant, essential for reducing surface tension in the alveoli, and can also mature into type I pneumocytes.
3. Fibrotic Phase
The majority of patients with ARDS recover their lung function within approximately 3 to 4 weeks. However, a small proportion of individuals progress to the fibrotic phase. These patients may require long-term support with mechanical ventilators and/or supplemental oxygen. In this phase, there is widespread scarring (fibrosis) affecting the alveolar ducts and the interstitial tissue of the lungs. Significant disruption of the normal structural organization of the lung units (acini) leads to changes resembling emphysema, characterized by the formation of large air-filled sacs called bullae. Within the small blood vessels of the lungs (pulmonary microcirculation), the inner lining thickens and proliferates (intimal fibroproliferation), leading to progressive blockage of these vessels and the development of pulmonary hypertension (high blood pressure in the lungs). The physiological consequences of this phase include an increased susceptibility to lung rupture (pneumothorax), a further reduction in lung compliance, and an increase in the portion of the lung that is ventilated but not involved in gas exchange (increased pulmonary dead space).
Signs and Symptoms
Infant respiratory distress syndrome typically starts soon after birth. Observable signs include:
Fast breathing (Tachypnea): An increased rate of respiration.
Fast heart rate (Tachycardia): An elevated heart rate.
Chest wall retractions (recession): The skin between the ribs pulls inward during breathing, indicating increased effort.
Expiratory grunting: A noise made during exhalation as the infant tries to keep the airways open.
Nasal flaring: The widening of the nostrils with each breath, a sign of respiratory distress.
Cyanosis: A bluish discoloration of the skin or mucous membranes due to low oxygen levels in the blood.
Ventilatory failure: As the condition worsens, there is a rise in carbon dioxide levels in the blood.
Prolonged cessations of breathing (“apnea”): Periods where breathing stops for an extended time.
Reduced urine output: Can occur as the body tries to conserve fluids.
Diagnosis/Investigation
Diagnosis typically involves assessing:
Signs and symptoms: Observing the clinical manifestations of the condition.
Chest x-ray: Imaging of the lungs to identify characteristic patterns associated with ARDS.
Pulse Oximetry: A non-invasive method to measure the oxygen saturation in the blood.
Echocardiography: An ultrasound of the heart to rule out cardiac causes of respiratory distress.
CT scans: More detailed imaging of the lungs.
Arterial blood gas (ABG) test: Measures the levels of oxygen, carbon dioxide, and acidity in the blood.
Differential Diagnosis
It’s important to differentiate ARDS from other conditions with similar symptoms:
Acute Anemia
Aspiration Syndromes
Pediatric Gastroesophageal Reflux
Pediatric Hypoglycemia
Pediatric Pneumonia
Pediatric Polycythemia
Pneumomediastinum
Pneumothorax
Transient Tachypnea of the Newborn
Management / Treatment
Treatment strategies include:
Delivery and resuscitation: In cases of premature birth, a neonatologist experienced in resuscitating premature infants should be present.
Keep the child warm: Maintaining body temperature is crucial.
Oxygen is given with a small amount of continuous positive airway pressure (CPAP): Helps to keep the airways open.
I.V. fluids: Solutions like normal saline (N/S), dextrose 5% (D5%), and balanced electrolyte solutions (e.g., Neonatalyte, Ringer’s Lactate) are administered to stabilize blood sugar, electrolytes, and blood pressure. Specific formulations may be used in neonates.
In severe cases, mechanical ventilation: An endotracheal tube is inserted, and a machine provides intermittent breaths.
Surfactant administration: Preparations of surfactant are given through the breathing tube to replace the missing natural surfactant in the lungs.
Administer a glucocorticoid: For example, dexamethasone, to reduce inflammation. Dosage is typically based on weight with a maximum dose.
Give an antibiotic: To prevent secondary bacterial infections.
Respiratory monitoring: Closely track breathing patterns.
Pulse rate, Bp, temperature, ECG monitoring: Continuous monitoring of vital signs.
Monitor conscious level: Assessing the patient’s level of alertness.
Reassure the mother: Providing emotional support.
NG tube feeding: Providing nutrition through a nasogastric tube.
Vitamin k: Administered intramuscularly to prevent bleeding, especially the risk of intraventricular hemorrhage.
Prevention
Preventive measures include:
Giving the mother glucocorticoids: This accelerates surfactant production in the fetus and is recommended for women at risk of preterm delivery before 34 weeks of gestation, with specific dosage guidelines.
Early antenatal care: Regular check-ups during pregnancy.
Eat healthy diet rich in vitamins: Promoting overall maternal health.
Avoid smoking and alcohol during pregnancy: These substances can negatively impact fetal lung development.
Complications
Potential complications of ARDS include:
Metabolic disorders: Such as acidosis and low blood sugar (hypoglycemia).
Patent ductus arteriosus (PDA): A condition where a blood vessel between the aorta and pulmonary artery doesn’t close properly after birth.
Low blood pressure (Hypotension):
Chronic lung changes: Long-term lung problems can develop.
Bleeding in the brain (Intraventricular hemorrhage): A risk, especially in premature infants.
CASE SCENARIO
1. An infant, one day old, is transferred to the ICU due to increased respiratory effort. Born prematurely at 31 weeks to a mother with a history of preterm deliveries, substance abuse, and HIV. The infant presents with elevated temperature, rapid heart rate, fast breathing, low blood pressure, and low oxygen saturation despite being on room air. Physical examination reveals tachypnea, nasal flaring, and subcostal retractions. Oxygen supplementation and positive pressure ventilation improve oxygen saturation. Blood glucose is within normal limits. Blood gas analysis reveals low pH, high carbon dioxide levels, and low oxygen levels. These findings strongly suggest a primary issue with the infant’s developing lungs and surfactant production.
2. A 55-year-old male presents with shortness of breath, fever, and cough. A chest x-ray indicates pneumonia in the right lower lobe, and he is started on intravenous antibiotics. The following day, he develops low oxygen levels (hypoxia) and low blood pressure (hypotension). His hypotension persists despite interventions like intubation, IV fluids, and vasopressors, leading to a diagnosis of septic shock. A subsequent chest x-ray shows new opacities in both lungs (bilateral alveolar opacities). An echocardiogram rules out heart failure. Arterial blood gas analysis reveals a low PF ratio, indicating severe impairment of gas exchange in the lungs due to widespread inflammation and fluid accumulation.
3. An infant, Dona, delivered via Cesarean section at 36 weeks’ gestation with a good Apgar score at birth, develops rapid breathing (tachypnea), chest wall retractions with nasal flaring, and a rapid heart rate (tachycardia) a few hours after delivery. Aside from the increased work of breathing, her physical examination is otherwise normal. A chest x-ray reveals a diffuse reticulogranular “ground glass” appearance with visible air bronchograms, a classic finding in neonatal respiratory distress syndrome due to surfactant deficiency and alveolar collapse.
Detailed Review.
The scenarios described are indicative of respiratory distress syndrome.
To understand this better, consider normal lung function. During inhalation, air reaches the alveoli, which are lined by two types of cells called pneumocytes. Type I pneumocytes are thin cells with a large surface area, crucial for gas exchange. Type II pneumocytes are smaller, thicker, and can multiply in response to lung injury. Their primary function is to produce surfactant, a fluid containing phospholipids that coats the inner surface of the alveoli. Surfactant acts like oil droplets, reducing surface tension, preventing the alveoli from collapsing upon exhalation. These type II cells can also differentiate into type I pneumocytes.
Acute respiratory distress syndrome (ARDS) is characterized by a rapid onset of widespread inflammation in the lungs, potentially leading to respiratory failure. ARDS is typically secondary to other conditions such as sepsis, aspiration, trauma, or pancreatitis.
The initiation of ARDS involves alveolar damage triggered by these underlying conditions. A key event is the release of inflammatory cytokines, such as TNF-alpha and interleukin-1, by the damaged pneumocytes. This cytokine release leads to the recruitment of neutrophils, which subsequently release toxic mediators like reactive oxygen species and proteases, further damaging the lung tissue.
The primary site of injury is the alveolar-capillary membrane, which becomes more permeable. This increased permeability allows fluid to leak into the alveoli, resulting in pulmonary edema. This fluid accumulation impairs gas exchange, leading to hypoxemia. Furthermore, the edema can wash away the surfactant, reducing its ability to lower surface tension, causing the alveoli to collapse. Over time, dead cells and protein-rich fluid accumulate in the alveolar spaces, forming waxy hyaline membranes, which appear as a glassy layer.
Individuals with ARDS exhibit serious symptoms requiring immediate attention. The inflammatory process and impaired gas exchange result in fever, shortness of breath, rapid breathing (tachypnea), chest pain, low blood pressure (hypotension), low oxygen levels (hypoxia), and bluish discoloration of the skin (cyanosis). Hypotension can frequently lead to shock. Auscultation of the lungs may reveal crackling sounds (rales), caused by collapsed alveoli popping open during inspiration. Additional symptoms might provide clues to the underlying cause; for example, upper abdominal pain radiating to the back with a history of gallstones suggests acute pancreatitis.
Diagnosis of ARDS is based on four main criteria. First, the symptoms must have an acute onset, typically within one week. Second, a chest X-ray or CT scan will show opacities or a “white out” appearance in both lungs due to pulmonary edema. Third, the PF ratio, calculated by dividing the partial pressure of oxygen in arterial blood by the fraction of inspired oxygen, will be below 300 mmHg, indicating impaired gas exchange. Lower PF ratios indicate more severe ARDS. Fourth, the respiratory distress cannot be primarily due to cardiac causes, such as heart failure. This is often assessed using echocardiography to evaluate heart function, looking for evidence of reduced ejection fraction (in systolic heart failure) or abnormal myocardial relaxation (in diastolic heart failure). Another differentiating factor is the pulmonary capillary wedge pressure, measured with a catheter. In heart failure, this pressure is elevated due to increased blood volume in the left side of the heart, whereas in ARDS, the pressure is typically normal because the edema is caused by leaky capillaries rather than increased pressure.
The primary approach to treating ARDS involves addressing the underlying condition. However, immediate supportive care is crucial, including supplemental oxygen or mechanical ventilation. Maintaining positive end-expiratory pressure (PEEP) is vital to keep the alveoli from collapsing. Using low tidal volumes during mechanical ventilation helps prevent over-inflation of damaged alveoli. It is important to be aware that positive pressure ventilation can compress pulmonary vessels, potentially leading to pulmonary hypertension and decreased pulmonary venous return, which can worsen hypotension and reduce cardiac output.
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved Design & Developed by Opensigma.co

