Sexually Transmitted Infections (STIs)
Subtopic:
HIV/AIDS hepatitis
HIV/AIDS
Human Immunodeficiency Virus (HIV) is a virus that targets the body’s defence system, specifically attacking CD4 cells (T cells). These cells are critical components of the immune system, essential for fighting off infections.
If left unmanaged, HIV can progress to Acquired Immunodeficiency Syndrome (AIDS). AIDS represents the advanced stage of HIV infection, characterized by a severely compromised immune system.
HIV is classified as a lentivirus, meaning it has a slow and prolonged action, and belongs to the Retroviridae family (retroviruses). HIV’s mechanism of action involves invading helper T cells to replicate itself. This process weakens the body’s ability to combat infections. It’s important to note that HIV is the causative agent of AIDS, and currently, there is no known cure for HIV infection.
Types of HIV
HIV-1: This is the most globally prevalent and dominant form of HIV. It is responsible for the vast majority of HIV infections worldwide. HIV-1 is highly infectious and is further categorized into various subtypes or clades, designated by letters A through K. HIV-1 is the primary driver of the global HIV pandemic and tends to progress to AIDS more rapidly compared to HIV-2.
HIV-2: This type is less common, with its geographical focus mainly in West Africa. HIV-2 is generally less easily transmitted and typically progresses to AIDS at a slower rate than HIV-1. HIV-2 has fewer subtypes, labeled A through H.
Characteristics of HIV/AIDS
RNA Virus: HIV is an RNA-based virus. A key feature is its ability to convert its RNA genetic material into DNA using an enzyme called reverse transcriptase.
Receptor Binding Mechanism: The HIV virus possesses specific proteins on its outer surface. These proteins enable the virus to attach to specific receptors on the surface of host cells, which is the crucial first step in viral entry into the cell.
Heat Sensitivity: HIV is susceptible to heat and is readily inactivated or destroyed by elevated temperatures, specifically around 60°C (140°F).
Human-Specific Host: HIV is specific to humans. It can only survive and multiply within human hosts. Outside the human body or after the death of the host, the virus cannot survive or replicate.
Immune System Target: HIV’s primary target is the immune system. It selectively attacks and destroys white blood cells, with a particular focus on CD4+ T cells, which are central to immune function.
Rapid Replication Rate: HIV is characterized by its high replication rate. It can produce billions of new viral particles (virions) every day within an infected individual.
Progressive Disease Course: HIV infection is not static; it follows a progressive course through distinct stages. If left untreated, it ultimately leads to AIDS, the most advanced and severe stage of the infection.
Latency Potential: Following the initial infection, HIV can enter a latent phase. In this phase, the virus can become dormant or hidden within cells, remaining inactive for a period before potentially reactivating at a later time.
Genetic Diversity and Mutation: HIV exhibits significant genetic diversity, with numerous subtypes and a high mutation rate. This genetic variability and rapid mutation pose challenges for both treatment strategies and vaccine development.
Immune Evasion Strategies: HIV has evolved mechanisms to evade the body’s immune responses. These strategies include rapid mutation, which allows the virus to change its surface proteins and escape immune recognition, and latency, which allows it to hide from immune surveillance within cells.
Transmission Routes: HIV is transmitted through direct contact with specific infected bodily fluids. These fluids include blood, semen, vaginal secretions, and breast milk. For transmission to occur, there must be a route for these fluids to enter the bloodstream or mucous membranes of an uninfected person.
Antiretroviral Therapy (ART): Antiretroviral therapy (ART) is a crucial treatment approach for HIV infection. ART does not cure HIV, but it is highly effective in controlling the virus. By suppressing viral replication, ART prevents the progression of HIV to AIDS and allows individuals with HIV to live longer and healthier lives.
Epidemiology
According to the Uganda Population-Based HIV Impact Assessment from August 2017, HIV prevalence in Uganda among adults aged 15-64 was 6.2%. Notably, prevalence was higher among females (7.6%) compared to males (4.7%). This translates to approximately 1.2 million adults in this age group living with HIV in Uganda. Urban women showed a higher HIV prevalence (9.8%) than rural women (6.7%).
Among children aged 0-14 years, HIV prevalence was 0.5%, representing around 95,000 children living with HIV. Viral load suppression (VLS), indicating effective treatment, among HIV-positive adults was 59.6%, with females showing a higher rate (62.9%) than males (53.6%). For children aged 0-14, the VLS rate was lower, at 39.3%.
HIV prevalence reached its peak at 14.0% among men aged 45-49 and at 12.9% among women aged 35-39. A significant gender disparity is evident in young adults. HIV prevalence was almost four times higher in females compared to males in the 15-19 and 20-24 age groups. Furthermore, HIV prevalence was almost three times higher in adults aged 20-24 compared to the 15-19 age group, highlighting increased risk in early adulthood.
Modes of HIV Transmission
HIV transmission occurs through specific routes involving direct contact with infected bodily fluids.
Sexual Contact:
Unprotected Vaginal Intercourse: HIV can be transmitted through the exchange of vaginal fluids and semen during vaginal sex without barrier protection (condoms).
Blood-to-Blood Contact:
Sharing Injection Equipment: Sharing needles, syringes, or other injection equipment, a common practice among people who inject drugs, is a high-risk route for HIV transmission.
Blood Transfusions: While now uncommon in regions with rigorous blood screening protocols, HIV can be transmitted through transfusions of infected blood or blood products if screening procedures are inadequate.
Occupational Exposure: Healthcare professionals are at risk of exposure to HIV through accidental needle-stick injuries or direct contact with infected blood through open wounds or mucous membranes.
Mother-to-Child Transmission (Vertical Transmission):
During Pregnancy: HIV can cross the placenta from an infected mother to her foetus during pregnancy.
During Childbirth: Infants can be exposed to HIV present in the mother’s blood and vaginal fluids during the process of vaginal delivery.
Breastfeeding: HIV can be transmitted through breast milk from an HIV-positive mother to her infant during breastfeeding.
Other Modes:
Contaminated Medical Equipment: The use of non-sterilized medical or dental instruments can potentially transmit HIV if these instruments become contaminated with infected blood.
Organ and Tissue Transplantation: Transplantation of organs or tissues from an HIV-infected donor can transmit HIV to the recipient. However, this is rare due to routine screening of donors.
Less Common Modes:
Tattooing and Body Piercing: If unsterilized needles and equipment are used for tattooing or body piercing, there is a risk of HIV transmission.
Contact Sports: Although exceptionally rare, HIV transmission theoretically could occur in contact sports if both individuals involved have open wounds that come into direct contact with infected blood.
Factors That Increase Mother-to-Child Transmission of HIV
Several factors can increase the likelihood of HIV transmission from a mother to her child. These can be broadly categorized as maternal, labour and delivery related, and postnatal feeding factors.
Maternal Factors:
Viral Load and Immune Status:
High Maternal Viral Load: Elevated levels of HIV in the mother’s bloodstream are directly correlated with a higher risk of transmission to the infant.
Low Maternal CD4 Count: A weakened maternal immune system, indicated by low CD4 cell counts, is associated with an increased risk of transmission.
Recent Maternal HIV Acquisition: Mothers who acquire HIV infection during pregnancy or while breastfeeding have a significantly higher risk of transmitting the virus to their child.
Infections and Inflammation:
Maternal Vaginal Infections: The presence of vaginal infections, such as bacterial vaginosis, can increase the risk of HIV transmission during delivery.
Chorioamnionitis: Inflammation of the foetal membranes due to infection (chorioamnionitis) can also facilitate HIV transmission from mother to child.
Access to and Adherence to Antiretroviral Therapy (ART):
Lack of Maternal ART: Mothers who do not receive ART during pregnancy have a substantially higher risk of transmitting HIV to their infants.
Poor Maternal ART Adherence: Inconsistent or inadequate use of ART by the mother reduces its effectiveness in preventing vertical transmission.
Late or Non-Initiation of Maternal ART: Starting ART late in pregnancy, or not at all, diminishes the preventive benefits of treatment.
Socioeconomic Factors:
Limited Healthcare Access: Restricted access to prenatal care services and HIV testing can result in missed opportunities for implementing preventive measures.
Limited Maternal Education and Awareness: Lack of knowledge among pregnant women regarding HIV transmission risks and effective prevention strategies can increase transmission rates.
Maternal Nutritional Status:
Poor Maternal Nutrition: Maternal malnutrition and nutritional deficiencies can weaken the mother’s immune system, potentially increasing the risk of HIV transmission.
Labour and Delivery Factors:
Delivery Method:
Vaginal Delivery: Vaginal delivery is associated with a higher risk of HIV transmission to the infant compared to elective Caesarean section, especially if the mother has a high viral load at the time of delivery.
Prolonged or Complicated Labour: Extended or difficult labour can increase the infant’s exposure to maternal bodily fluids, potentially raising the risk of transmission.
Prematurity:
Premature Birth: Premature infants have underdeveloped immune systems, which can make them more susceptible to HIV infection if exposed.
Prolonged Rupture of Membranes (PROM):
PROM Duration: Prolonged rupture of amniotic membranes (PROM) lasting for more than 4 hours before delivery increases the risk of HIV transmission due to increased exposure of the infant to the vaginal canal and potential ascending infection.
Invasive Monitoring and Procedures:
Intrapartum Interventions: The use of invasive foetal monitoring techniques or other invasive procedures during labour and delivery may slightly elevate the risk of HIV transmission.
Postnatal Feeding Factors:
Breastfeeding Practices:
Duration of Breastfeeding: Extended durations of breastfeeding are associated with a cumulative increase in the risk of HIV transmission over time.
Maternal Breast Health Issues: Breast health problems in the mother, such as sore nipples, breast abscesses, or mastitis, can elevate the risk of HIV transmission through breastfeeding.
Mixed Infant Feeding: Combining breastfeeding with other foods or fluids (mixed feeding) is associated with an increased risk of HIV transmission compared to exclusive breastfeeding or exclusive formula feeding. Exclusive breastfeeding for the first 3-6 months has not been shown to significantly increase transmission risk compared to formula feeding alone, when ART is in place and adhered to.
Exclusive Breastfeeding:
Definition: Exclusive breastfeeding is defined as providing only breast milk to the infant, with no other liquids, water, foods, teats, or pacifiers. It involves feeding the infant on demand whenever the baby shows signs of hunger.
Infant Oral Health:
Oral Thrush in Infants: The presence of oral thrush (Candida infection in the mouth) in breastfed infants has been suggested to potentially increase the risk of HIV transmission.
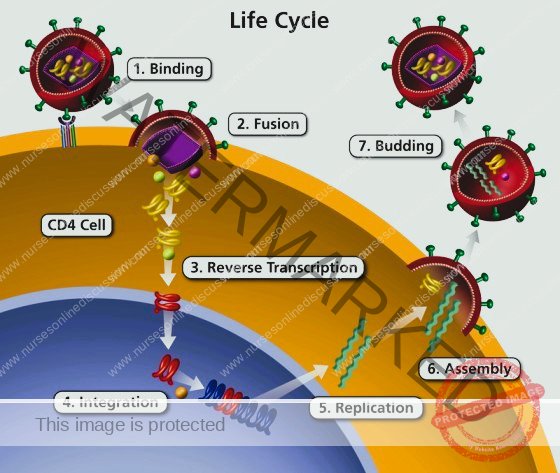
Phases of HIV Entry into Host Cells
HIV infection of a host cell is a multi-step process that allows the virus to hijack cellular machinery for its own replication. These phases are crucial targets for antiretroviral therapies.
Attachment (Binding): The HIV virus initiates infection by specifically attaching to a host cell. This process begins with the gp120 protein on the HIV envelope recognizing and binding to the CD4 receptor on the surface of the target cell. The primary target cells are typically CD4+ T lymphocytes, which are critical components of the immune system. This initial CD4 receptor interaction induces a change in the shape of gp120, enabling it to interact with a co-receptor. These co-receptors are usually CCR5 or CXCR4, also located on the host cell surface. This dual receptor binding – to both CD4 and a co-receptor – is a mandatory step for HIV to proceed with infecting the cell.
Membrane Merging (Fusion): Following successful binding, the HIV viral envelope needs to merge with the host cell membrane to deliver its contents inside. The conformational shift in gp120, triggered by the CD4 and co-receptor binding, exposes another HIV protein called gp41. gp41 facilitates the actual fusion event. It promotes the merging of the viral envelope with the host cell membrane, creating a channel or fusion pore. Through this pore, the viral core, known as the capsid, containing the viral RNA and essential enzymes, is released into the host cell’s cytoplasm.
RNA to DNA Conversion (Reverse Transcription): Once inside the host cell, the viral RNA genome must be converted into DNA, as host cells use DNA as their genetic material. This conversion is carried out by an enzyme called reverse transcriptase, which is brought into the cell within the viral capsid. Reverse transcriptase catalyses the process of reverse transcription, converting the single-stranded viral RNA into double-stranded viral DNA. This is a unique and error-prone process, leading to frequent mutations in the viral genome. This high mutation rate is a key factor in HIV’s ability to evade the immune system and develop resistance to antiviral drugs.
Genetic Material Integration (Integration): The newly synthesized viral DNA must become a permanent part of the host cell’s genetic makeup. The viral DNA is transported into the nucleus of the host cell, which houses the host cell’s DNA. Here, another viral enzyme, integrase, mediates the integration of the viral DNA into the host cell’s DNA. The integrated viral DNA is now called a provirus. The provirus can remain in a latent or dormant state within the host cell’s genome for an extended period, potentially becoming active later.
Viral Component Production (Replication): Once integrated and activated, the viral DNA is used to direct the production of new viral components. The host cell’s own cellular machinery is hijacked for this purpose. The host cell begins to transcribe the integrated viral DNA into viral RNA molecules. Some of these viral RNA copies will serve as the genomes for new viral particles. Other viral RNA molecules are translated into viral proteins through the process of translation, using the host cell’s ribosomes.
New Virus Particle Assembly (Assembly): New viral particles are constructed within the host cell. The newly synthesized viral RNA genomes and viral proteins are transported to the host cell’s plasma membrane. At the cell surface, these components begin to assemble into new, immature viral particles. This assembly process involves the coming together of all the necessary viral building blocks to form a new virion.
Release and Maturation (Budding): The newly assembled, immature viral particles are released from the host cell. These immature particles bud off from the host cell membrane. During budding, the new viral particle becomes enclosed by a portion of the host cell’s plasma membrane, which forms the viral envelope. At this stage, the viral particles are still immature and non-infectious. The final step is maturation, which occurs after budding. A viral enzyme called protease becomes active and cleaves certain large viral precursor proteins into smaller, mature proteins. This proteolytic processing is essential for the virus to become fully mature and infectious, ready to infect new host cells and continue the viral life cycle.
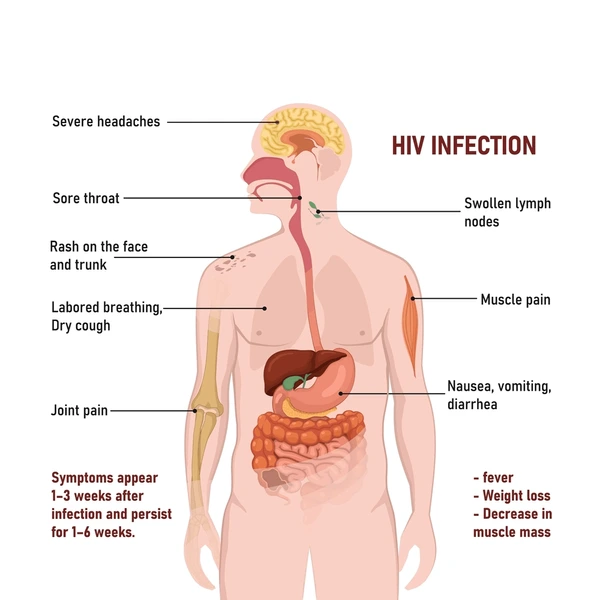
Clinical Manifestations of HIV/AIDS
The World Health Organization (WHO) has developed a clinical staging system to categorize HIV infection and its progression.
Clinical Stage I:
Asymptomatic Phase: Individuals in this stage show no noticeable signs or symptoms directly related to HIV infection.
Persistent Generalized Lymph Node Enlargement: Characterized by the lasting enlargement of lymph nodes in multiple sites throughout the body, persisting for over three months. This indicates an active immune response, though non-specific to HIV.
Performance Status 1: Patients are asymptomatic and maintain a normal level of physical activity without functional limitations.
Clinical Stage II:
Moderate Weight Reduction: Unintentional weight loss amounting to less than 10% of the individual’s baseline or expected body weight.
Minor Skin and Mucous Membrane Issues: Presence of less severe conditions affecting the skin and mucous membranes, such as:
Seborrheic dermatitis: A common skin condition causing scaly patches and redness, often on the scalp and face.
Prurigo: An intensely itchy skin rash characterized by small bumps.
Onychomycosis (Fungal Nail Infections): Fungal infections affecting the nails, causing thickening, discoloration, and brittleness.
Herpes Zoster History: Reported occurrence of shingles (Herpes Zoster) within the past five years. Shingles is a reactivation of the chickenpox virus causing a painful rash.
Recurrent Upper Respiratory Infections: Experiencing repeated infections of the upper airways, for example:
Bacterial sinusitis: Infection of the sinuses.
Tonsillitis: Inflammation of the tonsils.
Otitis media: Middle ear infection.
Performance Status 2: Individuals are symptomatic but still capable of maintaining a normal activity level, although they may experience some symptoms.
Clinical Stage III:
Significant Weight Loss: Marked weight loss exceeding 10% of the individual’s usual or anticipated body weight.
Unexplained Chronic Diarrhoea: Persistent diarrhoea with no identifiable cause, lasting for more than a month.
Unexplained Prolonged Fever: Recurring or continuous fever without a clear origin, persisting for over one month.
Oral Candidiasis (Thrush): A fungal infection in the mouth caused by Candida, presenting as whitish patches.
Oral Hairy Leukoplakia: Distinctive white, hairy-appearing patches on the sides of the tongue or inside the mouth, often linked to viral infection.
Pulmonary Tuberculosis (TB): Active infection of the lungs with tuberculosis bacteria.
Severe Bacterial Infections: Serious bacterial infections, including but not limited to:
Pneumonia: Lung infection.
Pyomyositis: Bacterial infection of the muscles.
Bacteremia: Presence of bacteria in the bloodstream.
Acute Necrotizing Ulcerative Gingivitis: A severe form of gum disease characterized by rapid tissue destruction, pain, and bleeding.
Unexplained Blood Disorders: Unexplained abnormalities in blood cell counts:
Anaemia: Low red blood cell count.
Neutropenia: Low neutrophil (a type of white blood cell) count.
Thrombocytopenia: Low platelet count.
Performance Status 3: Patients are bedridden for less than half of the day (under 50%) during the preceding month, indicating a reduced functional capacity.
Clinical Stage IV:
HIV Wasting Syndrome: Defined by significant weight loss (over 10%) accompanied by either chronic diarrhoea or prolonged fever.
Pneumocystis Pneumonia (PCP): A serious lung infection caused by the fungus Pneumocystis jirovecii, a major opportunistic infection in AIDS.
Cerebral Toxoplasmosis: An infection of the brain caused by the parasite Toxoplasma gondii.
Cryptosporidiosis: An intestinal infection caused by Cryptosporidium parasites, leading to persistent and severe diarrhoea.
Cytomegalovirus (CMV) Infection: A viral infection that can affect various organs, including the eyes (retinitis), lungs, and gastrointestinal tract, especially in immunocompromised individuals.
Progressive Multifocal Leukoencephalopathy (PML): A severe brain infection caused by the JC virus, leading to progressive neurological damage and symptoms.
Lymphoma: Cancers originating in the lymphatic system. Certain types are more common and aggressive in individuals with advanced HIV.
Kaposi’s Sarcoma (KS): A type of cancer causing skin lesions and potentially affecting internal organs, caused by Human Herpesvirus-8 (HHV-8).
HIV Encephalopathy (AIDS Dementia Complex): Neurological impairment characterized by cognitive and motor dysfunction directly resulting from HIV infection of the brain.
Atypical Disseminated Leishmaniasis: A widespread form of Leishmaniasis, a parasitic disease, affecting multiple organs in an unusual pattern in HIV-infected individuals.
Symptomatic HIV-Associated Nephropathy or Cardiomyopathy: Presence of kidney disease (nephropathy) or heart muscle disease (cardiomyopathy) that is directly linked to HIV infection and causing clinical symptoms.
Performance Status 4: Individuals are largely incapacitated and bedridden for more than half of the day (over 50%) during the last month, indicating a significant decline in functional status.
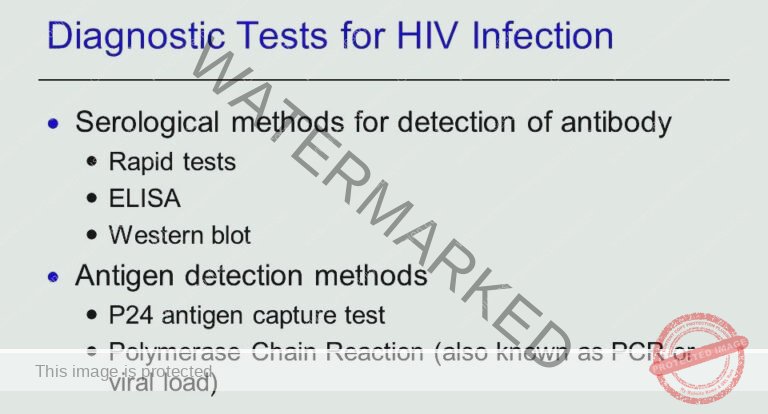
Diagnostic Measures for HIV/AIDS
Pre- and Post-Test Counselling and Informed Consent: Counselling and obtaining informed consent are fundamental before any HIV diagnostic procedure. However, exceptions exist in specific situations:
Clinical Urgency: Testing may be conducted by healthcare teams without explicit consent for patients who are critically ill, unconscious, symptomatic of AIDS, or have impaired mental capacity, when the test results are essential for optimal patient management.
Public Health Necessity: Routine HIV testing is indicated for individuals who are highly likely to transmit HIV to others. This includes:
Pregnant and breastfeeding women (for prevention of mother-to-child transmission).
Individuals involved in sexual offenses (both offenders and survivors).
Prospective blood and organ donors (to ensure blood and organ safety).
Even in these routine testing scenarios, individuals should be informed of their right to know their HIV status and given the opportunity to receive their results and appropriate support.
Diagnostic Criteria: HIV/AIDS diagnosis is established based on:
Clinical Stage Assessment: Evaluation using the WHO Clinical Staging Criteria for HIV infection.
Confirmed Positive HIV Blood Test: Laboratory verification of HIV infection through a positive HIV serological (antibody) blood test.
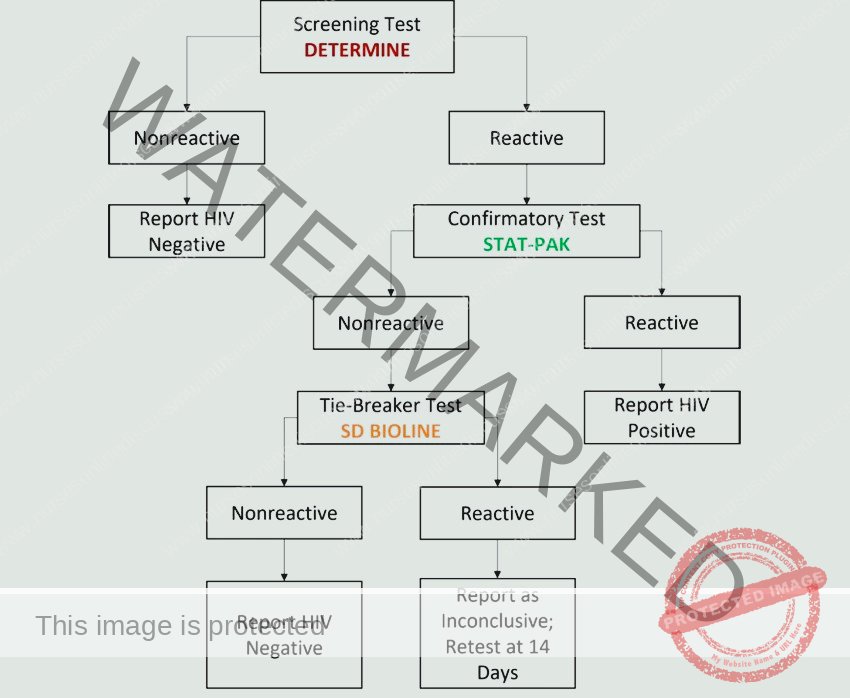
HIV Testing Protocol for Adults and Children Over 18 Months:
Serological (Antibody) Testing: This is the most frequently employed method for HIV diagnosis.
Window Period Consideration: It’s crucial to understand the “window period”—the time between HIV infection and the body producing detectable antibodies. If an individual tests negative but has had recent potential HIV exposure, repeat testing is recommended after three months to definitively rule out infection.
Reactive Rapid Test and Confirmation: A reactive (positive) result from a rapid HIV antibody test requires laboratory confirmation with a supplementary test to ensure accuracy before a definitive diagnosis is made.
Diagnostic Tests in Detail:
Screening Tests:
Enzyme-Linked Immunosorbent Assay (ELISA) Ag/Ab Assays: These assays are widely used as screening tests, particularly for blood donations. They are designed to detect both HIV antibodies and antigens (viral proteins), which helps to identify infections even during the window period when antibodies alone may not be detectable.
Molecular Tests:
Polymerase Chain Reaction (PCR) Tests (Nucleic Acid Amplification Testing – NAT): PCR-based NAT tests are highly sensitive molecular assays. Instead of detecting antibodies or antigens, they directly detect HIV’s genetic material (RNA or DNA). These tests can identify HIV infection very early, even before antibody production, and are valuable in specific situations like early infant diagnosis or confirming indeterminate antibody tests.
Key Considerations for Testing:
Contextual Approach: HIV testing should always be performed considering the individual’s:
Current clinical presentation and symptoms.
Relevant medical history.
Individual risk factors for HIV exposure.
Integrated Assessment: Test results should be interpreted in conjunction with a comprehensive patient assessment to ensure accurate diagnosis and guide appropriate patient care and management strategies.
Immediate Linkage to HIV Care:
Prompt Referral: For any individual who tests positive for HIV, immediate referral to dedicated HIV care services is paramount. This ensures timely initiation of appropriate medical management, including antiretroviral therapy (ART) and access to comprehensive support services.
HIV Testing Provision Protocol
Step 1: Pre-Test Information and Counseling
Provide comprehensive information on HIV transmission routes, effective prevention strategies, and the benefits of HIV testing.
Discuss potential test outcomes, available support services, and ensure informed consent and maintain strict confidentiality.
Perform individual risk assessments to understand exposure risks and complete all required documentation.
Step 2: HIV Testing
Conduct blood-based HIV testing using appropriate methods.
For infants under 18 months, utilize DNA PCR testing for accurate early diagnosis.
For individuals over 18 months, perform antibody testing following established testing algorithms for reliable results.
Step 3: Post-Test Counseling (Individual/Couple)
Assess the individual’s readiness to receive test results and communicate results clearly and sensitively.
Address any concerns, provide guidance on safe disclosure practices, partner testing options, and personalized risk reduction strategies.
Offer detailed information on essential HIV care services, the benefits of Antiretroviral Therapy (ART), and ensure complete and accurate documentation.
Step 4: Linkage to Other Services
Provide detailed information on available HIV-related services and actively assist in completing necessary referral forms to facilitate access.
Upon successful enrollment in services, meticulously record pre-ART enrollment numbers and ensure seamless transfer of relevant information to ART registers for continuity of care.
Principles of HIV Testing Services (HTS)
Confidentiality: Maintain strict privacy and confidentiality of all test results and personal information.
Consent: Always obtain informed consent from individuals prior to conducting any HIV testing.
Counselling: Offer supportive and informative counselling services both before and after HIV testing to aid understanding and coping.
Correct Test Result: Ensure the accuracy and reliability of test results through adherence to proper testing procedures and quality control measures.
Connection to Other Services: Facilitate seamless access to appropriate prevention, care, and treatment services for individuals based on their test results and needs.
Linkage from HIV Testing to Prevention, Care, and Treatment
Linkage is the critical process of connecting individuals newly diagnosed with HIV to essential prevention, care, and treatment services.
Effective linkage to care is crucial for ensuring that individuals living with HIV promptly access and receive the necessary services for their health and well-being. For HIV-positive individuals, linkage should be expedited, ideally within seven days if within the same healthcare facility, and within 30 days for referrals to external facilities or from community-based testing. Lay providers are often recommended as effective linkage facilitators to support this process.
Types of Linkages:
Internal Facility Linkage: Connecting patients to care services within the same healthcare facility where they were tested.
Inter-Facility Linkage: Referrals and connections established when patients need to be linked to care services at a different healthcare facility.
Community-Facility Linkage: Bridging the gap between community-based HIV testing services and formal healthcare facilities for ongoing care.
Internal Facility Linkage Steps:
Post-Test Counselling: Deliver accurate test results and provide comprehensive information about available care and support options within the facility.
Next Steps Discussion: Clearly explain the process of HIV care and treatment, emphasizing the significant benefits of initiating treatment early.
Address Barriers to Linkage: Proactively identify and address any potential obstacles or barriers that might hinder the patient’s linkage to care services.
Patient and Family Involvement: Actively involve the patient and their family members (if appropriate and with consent) in the decision-making process regarding care and treatment.
Documentation and Paperwork: Ensure proper completion of all necessary client forms and referral documentation to facilitate seamless transition to care.
Escorted Referral to Clinic: A designated linkage facilitator personally escorts the client to the ART (Antiretroviral Therapy) clinic or relevant care unit within the facility.
Enrollment in Care: Facilitate patient registration, initiate the opening of an ART file, and provide preparatory counselling to prepare the patient for treatment.
ART Initiation: Commence Antiretroviral Therapy (ART) if the patient is assessed as ready and willing, and continue to provide ongoing counselling and support.
Integrated Care Services: Coordinate access to other necessary integrated services, such as TB screening, mental health support, or family planning, as needed.
Follow-Up and Appointment Reminders: Implement systems to ensure the patient attends scheduled appointments and receives ongoing follow-up care.
Inter-Facility and Community-Facility Linkages:
Inter-Facility Linkage: Involves connecting patients to care at a different facility. The referring facility should establish a tracking system to monitor referred patients and ensure successful enrollment in care within 30 days of referral.
Community-Facility Linkage: Focuses on connecting individuals diagnosed in community settings to formal health facilities. This approach utilizes community health workers and mobilizes peer leaders to conduct outreach, provide support, and ensure follow-up to facilitate linkage within 30 days post-diagnosis.
Treatment Modalities of HIV/AIDS
| Treatment Modality | Description |
| Antiretroviral Therapy (ART) | The cornerstone of HIV treatment, ART aims to suppress the viral load to undetectable levels, significantly reducing morbidity, mortality, and the risk of HIV transmission. |
| Treatment of Acute Bacterial Infections | Addresses any immediate bacterial infections that may occur, as individuals with HIV are more susceptible to various infections. |
| Prophylaxis and Treatment of Opportunistic Infections (OIs) | Employs preventative measures and treatments to manage opportunistic infections, which are infections that take advantage of weakened immune systems in people with HIV. |
| Maintenance of Good Nutrition | Emphasizes the importance of adequate nutrition to support overall health, immune function, and well-being in individuals living with HIV. |
| Immunization | Recommends and administers appropriate vaccines to prevent vaccine-preventable opportunistic infections, enhancing immune protection. |
| Management of AIDS-Defining Illnesses | Focuses on the specific management and treatment of AIDS-defining illnesses, which are severe conditions that indicate advanced HIV infection and require targeted medical interventions. |
| Psychological Support for the Family | Recognizes the psychosocial impact of HIV and provides essential emotional support, counselling, and guidance for affected individuals and their families to cope with the challenges of living with HIV. |
| Palliative Care for the Terminally Ill | Offers comprehensive comfort care, symptom management, and psychosocial support for patients in the advanced stages of AIDS, focusing on improving quality of life and providing compassionate end-of-life care. |
Antiretroviral Drug Treatment
Goal of ART: The primary goal of Antiretroviral Therapy (ART) is to achieve and maintain undetectable viral load. This significantly reduces HIV-related morbidity, mortality, and the risk of onward transmission.
When to Initiate ARV:
All HIV-infected children under 12 months of age: Early ART initiation is crucial for infants to improve survival and health outcomes.
Clinical AIDS (WHO Stage III or IV): Individuals diagnosed with clinical AIDS, regardless of CD4 count, should commence ART.
Mild to moderate symptoms and immunosuppression (WHO Stage I or II and/or CD4 count <350 cells/mm3): ART is recommended for those with symptoms or signs of immune compromise.
Process of Starting ART:
Opportunistic Infection Assessment: Screen for opportunistic infections (OIs), and if active TB or cryptococcal meningitis is present, ART initiation may be deferred until OI treatment is underway.
Offer Same-Day ART Initiation: Provide ART initiation on the same day of diagnosis through an opt-out approach, making ART readily accessible.
Timely ART Preparation Plan: If same-day initiation is not feasible or preferred by the patient, collaboratively agree on a timely and patient-centered plan to prepare for and start ART as soon as possible.
Available ARVs in Uganda:
| Drug Class | Examples |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | Tenofovir (TDF), Zidovudine (AZT), Lamivudine (3TC), Abacavir (ABC) |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | Efavirenz (EFV), Nevirapine (NVP), Etravirine (ETV) |
| Integrase Inhibitors (INIs) | Dolutegravir (DTG), Raltegravir (RAL) |
| Protease Inhibitors (PIs) | Atazanavir (ATV), Lopinavir (LPV), Darunavir (DRV) |
| Entry Inhibitors (Fusion Inhibitors and CCR5 antagonists) | Enfuvirtide (T-20), Maraviroc |
Recommended First-Line Regimens in Adults, Adolescents, Pregnant Women, and Children:
Note: HIV management guidelines are dynamic and subject to updates. Always consult the most recent official guidelines for current recommendations.
The 2022 Ugandan guidelines prioritize Dolutegravir (DTG), an integrase inhibitor, as the anchor drug in both first- and second-line regimens for all HIV-infected individuals, including children, adolescents, men, women (pregnant, breastfeeding, and of childbearing potential).
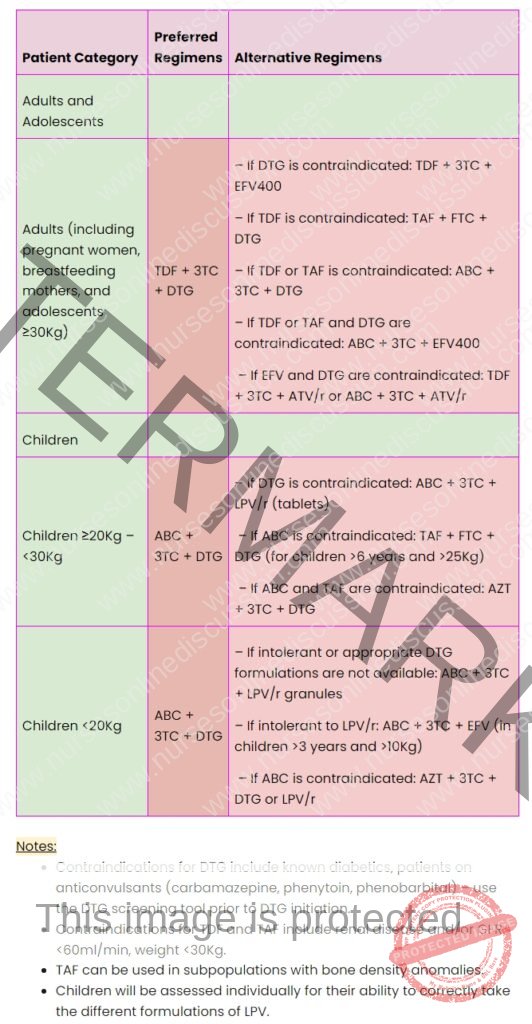
Notes on DTG and TDF/TAF Contraindications & Ministry of Health (Uganda) Guidance
Drug Contraindications:
Dolutegravir (DTG): DTG is contraindicated in individuals with known diabetes and those using certain anticonvulsant medications (carbamazepine, phenytoin, phenobarbital). A DTG screening tool should be utilized before initiating DTG therapy to identify potential risks.
Tenofovir (TDF) and Tenofovir Alafenamide (TAF): TDF and TAF are contraindicated in patients with pre-existing renal disease and/or a Glomerular Filtration Rate (GFR) below 60ml/min, and in individuals weighing less than 30Kg.
Tenofovir Alafenamide (TAF) Advantage: TAF may be considered for specific patient groups with bone density concerns.
Lopinavir/ritonavir (LPV/r) for Children: Children’s ability to correctly take various LPV/r formulations requires individual assessment.
Ministry of Health (Uganda) – Specific Notes:
ABC-3TC-DTG Fixed-Dose Combination (FDC): For clients on an Abacavir/Lamivudine/Dolutegravir regimen who weigh over 25 kg, it is recommended to use the fixed-dose combination tablet (600/300/50 mg) instead of administering separate pills of Abacavir/Lamivudine and Dolutegravir. This simplifies dosing and improves adherence.
Abacavir/Lamivudine 600/300 mg Use: Abacavir/Lamivudine combination pills (600/300 mg) should be used for patients on regimens including: ABC-3TC-ATV/r, ABC-3TC-LPV/r, and ABC-3TC-DRV/r. This ensures correct dosing when these regimens are prescribed.
Dolutegravir 50 mg Single Pill Use: For patients on AZT-3TC-DTG regimens, utilize the Dolutegravir 50 mg single pill formulation for accurate dosage.
Optimize to Dolutegravir: For patients currently on Atazanavir/ritonavir (ATV/r) and Lopinavir/ritonavir (LPV/r) based regimens, consider switching to Dolutegravir-based regimens if clinically appropriate and eligible. This reflects the preference for DTG in current guidelines.
Pre-Exposure Prophylaxis (PrEP) – TDF/FTC Preference: While guidelines allow for either TDF/3TC or TDF/FTC for PrEP, programmatic implementation should prioritize the use of TDF/FTC 300/200 mg for PrEP. This recommendation may be based on factors like efficacy and availability.
Monitoring of Antiretroviral Therapy (ART)
Purposes of ART Monitoring:
Treatment Response and Failure Detection: To evaluate how effectively ART is working and to identify if treatment is failing.
Medication Safety: To monitor for potential side effects and toxicities associated with ART drugs.
Adherence Assessment: To assess how well patients are adhering to their prescribed ART regimen.
Methods for Monitoring ART:
Clinical Monitoring: This involves regularly reviewing the patient’s medical history and conducting physical examinations to assess their clinical status and identify any new symptoms or signs.
Laboratory Monitoring: This utilizes various laboratory tests to objectively assess treatment response and identify potential issues.
Viral Load (VL) Monitoring: This is the preferred laboratory method for assessing ART response and diagnosing treatment failure.
CD4 Monitoring: CD4 cell count monitoring is recommended in specific clinical scenarios.
Other Minor Laboratory Tests: Additional tests may be indicated based on individual patient needs and potential drug-related issues.
Viral Load Monitoring Details:
Preferred Method: Viral load testing is the most effective way to monitor ART response.
Viral Suppression Target: For patients on ART for over 6 months and responding well, viral load should be suppressed to undetectable levels (typically defined as <1000 copies/mL), regardless of whether the sample is Dried Blood Spots (DBS) or plasma.
Early Detection of Treatment Failure: Viral load monitoring provides an early and accurate indication if treatment is failing, prompting timely switching to second-line ART regimens. This reduces the development of drug resistance mutations and improves patient outcomes.
Distinguishes Failure from Non-Adherence: Viral load results can help differentiate between true treatment failure (drug resistance) and non-adherence to medication.
Recommended Frequency: Viral load testing is generally recommended every six months for children and adolescents under 19 years of age.
CD4 Monitoring Details:
Baseline CD4 Count Importance: A baseline CD4 count at the start of ART is valuable for assessing the patient’s risk of opportunistic infections.
Specific Recommendations: CD4 monitoring is particularly recommended for patients presenting with a high viral load or advanced clinical disease.
Other Laboratory Tests
| Tests | Indication |
| CrAg Screen | Screening for cryptococcal infection |
| Complete Blood Count (CBC) | Assessing risk of anaemia |
| TB Tests | Suspected Tuberculosis (TB) |
| Serum Creatinine | Assessing kidney function |
| ALT, AST | Evaluating liver function |
| Lipid Profile, Blood Glucose | Assessing metabolic health (e.g., dyslipidemia, diabetes risk) |
Problems Associated with ARV Treatment
Immune Reconstitution Inflammatory Syndrome (IRIS)
Definition: IRIS is a condition characterized by a range of clinical signs and symptoms resulting from the recovering immune system’s response to pre-existing infections after starting ART.
Prevalence: IRIS occurs in a notable proportion of individuals initiating ART, estimated between 10% and 30%, typically within the first 4 to 8 weeks of treatment.
Serious Forms: The most severe IRIS cases are often seen in patients co-infected with Tuberculosis (TB), Cryptococcus, Kaposi’s sarcoma, and herpes zoster, as the immune system mounts a strong inflammatory response against these pathogens.
Risk Factors: Key risk factors for IRIS include having a low CD4+ cell count at ART initiation (below 50 cells/mm³) and the presence of disseminated opportunistic infections before starting ART.
Management: IRIS is usually a self-limiting condition. Management focuses on treating underlying co-infections to alleviate symptoms and providing reassurance to patients to encourage continued adherence to ART.
Steps to Minimize IRIS Development:
Early HIV Diagnosis and ART Initiation: Diagnosing HIV early and starting ART before the CD4 count drops significantly (ideally before it falls below 200 cells/mm³) can reduce IRIS risk.
Optimal Management of Opportunistic Infections: Screening for and treating opportunistic infections, particularly TB and cryptococcal infection, before initiating ART can also help to lower the risk of IRIS.
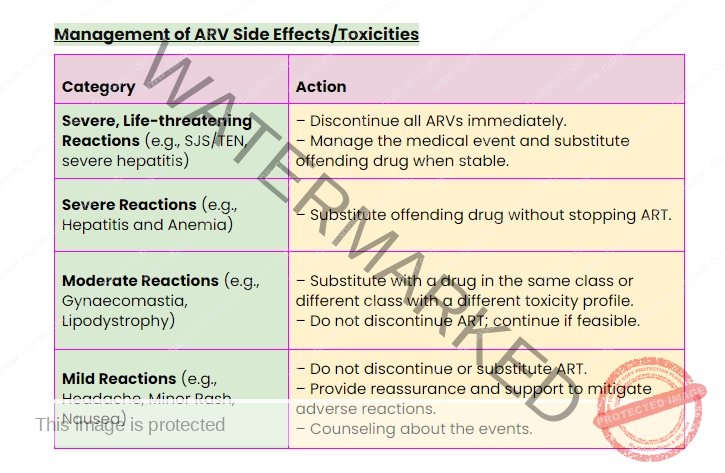
ARV Drug Toxicity
Spectrum of Toxicities: Antiretroviral drugs can cause a wide range of side effects, varying from mild and manageable to severe and life-threatening.
Diagnostic Challenges: Distinguishing between ARV drug toxicity and complications arising from HIV infection itself can be clinically challenging.
Management Strategy: Regularly assess patients for potential side effects at each clinic visit. Management actions should be guided by the severity of the reaction.
Management of ARV Side Effects/Toxicities
Adherence Preparation, Monitoring, and Support
Maintaining consistent adherence to Antiretroviral Therapy (ART) is paramount for achieving sustained viral suppression in individuals living with HIV. Optimal adherence is directly linked to reduced drug resistance and improved long-term health outcomes. Conversely, inconsistent or poor adherence to ART regimens is a major factor contributing to treatment failure. Therefore, routine assessment and reinforcement of adherence strategies by the healthcare team are vital aspects of comprehensive HIV care.
Adherence Preparation: Preparing individuals before they commence antiretroviral therapy is a critical step towards ensuring successful ART outcomes.
Initiation Discussion: Healthcare providers should actively engage patients in comprehensive conversations to ascertain their preparedness and willingness to begin ART. While healthcare professionals are responsible for providing essential information and guidance, the ultimate choice regarding ART initiation should be made by the patient themselves, or their caregiver if applicable.
The “5 As” Framework for Chronic Care: To deliver effective pre-ART adherence counselling and psychosocial support, the clinical team should utilize the “5 As” framework. This structured approach includes: Assess, Advise, Agree, Assist, and Arrange.
Key Steps in ART Preparation:
Assess: Evaluate the patient’s current level of understanding regarding HIV infection, antiretroviral medications, and potential challenges they might face in adhering to treatment.
Advise: Provide patients with pertinent and easily understandable information about ART. This empowers them to make informed decisions about enrolling in and committing to treatment.
Agree on: Collaboratively develop a personalized adherence plan with the patient. This should also include identifying and strengthening existing support networks, including family members and community resources.
Assist: Actively help patients identify and proactively address any potential barriers that could hinder their ability to adhere to the prescribed ART regimen.
Arrange for: Schedule crucial follow-up appointments, including the initial ARV prescription pick-up, subsequent counselling sessions focused on adherence, and connections to relevant psychosocial support groups or services.
Barriers to Adherence of ART
| Barrier | Adolescents | Pregnant or Breastfeeding Women | Adults | Key Populations |
| Psychosocial Issues | Peer Influence: Pressure from peers and the desire to fit in can hinder adherence. | |||
| Inconsistent Daily Routine | Present | |||
| Child Abuse and Neglect | Present | |||
| Stigma and Discrimination | Present | Present | Present | Present |
| Limited Involvement in Decision-Making | Present: Feeling excluded from choices and having few opportunities to discuss treatment. | |||
| Low Health Literacy & Counselling Access | Present: Limited understanding of treatment or lack of access to support services. | |||
| Challenges in Healthcare Transition | Present: Difficulties moving from children’s to adult healthcare services. | |||
| Pregnancy-Related Discomfort | Present: Conditions like nausea and vomiting during pregnancy. | |||
| Suboptimal HIV/ART Knowledge | Present: Inadequate understanding of HIV, ART, and eMTCT procedures. | |||
| Lack of Partner Disclosure/Support | Present: Not revealing status to partner or lacking partner support. | |||
| Non-Disclosure of Status | Present | Present | Present | |
| Gender-Based Violence (GBV) | Present | Present | Present | |
| Sharing Medication | Present | |||
| Service Delivery Obstacles | Present: Problems related to how services are provided. | |||
| Substandard Clinical Practices | Present: Poor quality of healthcare services received. | |||
| Provider Knowledge Gaps | Present: Healthcare providers lacking sufficient knowledge/training. | |||
| Limited Access to Services | Present: Difficulty in reaching or utilizing healthcare services. | |||
| Social Work-Life Conflicts | Present | |||
| Forgetfulness | Present | |||
| Distrust in Healthcare System | Present | |||
| Limited Community Support | Present | |||
| Medication Side Effects | Present | |||
| Pill Burden | Present | |||
| Insufficient ARV Information | Present | |||
| Alcohol and Substance Use | Present | Present | Present | |
| Negative Provider Attitude | Present | |||
| Frequent Relocation | Present | |||
| Lack of Peer Group Support | Present | |||
| Health Worker Knowledge Deficit (KPs) | Present |
Methods of Monitoring Adherence to ART
Viral Load Measurement: Considered the most reliable method for evaluating both patient adherence to ART and the effectiveness of the treatment. It is recommended to perform viral load testing six months after ART initiation and then annually for ongoing monitoring.
Patient Self-Reporting: A quick, inexpensive, and easily implemented approach, relying on patients’ accounts of their medication-taking behavior. However, it’s important to acknowledge that self-reports may be influenced by reporting bias and may not always be entirely accurate.
Pill Counts: Involves physically counting the remaining pills to estimate adherence. While simple to perform, pill counts have limitations as patients might discard pills before appointments to appear adherent. Combining pill counts with self-reported adherence can improve the reliability of this method.
Pharmacy Refill and Clinic Records Review: Examining pharmacy refill records and clinic attendance logs offers objective documentation of medication pick-up patterns and appointment keeping. This data can be a valuable indicator of potential adherence issues or patterns of non-adherence over time.
Adherence Support Strategies
To enhance and sustain adherence to ART, a range of support interventions should be made available to individuals on treatment, incorporating the following:
Peer Support Systems: Utilizing peer counselors, including peer mothers within eMTCT programs, adolescent peer mentors, experienced clients, and other peer advocates. Patients and caregivers often find peer-based support particularly helpful due to shared experiences and understanding.
Mobile Health (mHealth) Interventions: Employing mobile phone calls and text messages for reminders and support, but only with explicit patient or caregiver consent. It is crucial to obtain and use correct contact details to prevent unintentional privacy breaches when sending messages.
Reminder Aids: Providing and encouraging the use of reminder tools such as calendars, pill organizers (pill boxes), and medication diaries to assist clients in managing their medication schedule.
Behavioral Skills and Adherence Training: Offering structured training programs that utilize module-based interventions and strategies designed to improve essential life skills, attitudes, behaviors, and knowledge related to medication adherence and overall health management.
Fixed-Dose Combinations (FDCs) and Once-Daily Regimens: Whenever clinically appropriate and available, healthcare providers should prioritize prescribing fixed-dose combination medications as they simplify the regimen and reduce the daily pill burden for patients. Similarly, if recommended and accessible, once-daily regimens should be considered to further enhance convenience and adherence.
Treatment Buddies: Encouraging patients to identify a “treatment buddy”—a trusted individual who agrees to provide support. The buddy’s role includes reminding the client to take their medication at the correct times and providing reminders about upcoming clinic appointments and medication refills.
Peer-Led Support Groups: Facilitating group discussions among clients living with HIV. These peer dialogues provide a platform for sharing experiences, discussing adherence challenges, and collaboratively developing potential solutions and coping strategies.
Uses of Antiretroviral Therapy (ART)
Primary Treatment for HIV/AIDS: ART serves as the foundational treatment approach for managing HIV infection and AIDS. It effectively controls viral replication, reduces the viral load, and preserves and strengthens the immune system.
Prevention of Vertical Transmission (PMTCT): ART is a critical component of strategies to prevent mother-to-child transmission (PMTCT) of HIV. It significantly reduces the risk of HIV transmission from a pregnant or breastfeeding mother to her child during pregnancy, childbirth, and breastfeeding.
Post-Exposure Prophylaxis (PEP): ART is utilized as an emergency preventive measure for individuals who have experienced potential exposure to HIV. To be effective, PEP must be initiated as soon as possible, ideally within 72 hours of the exposure event.
Pre-Exposure Prophylaxis (PrEP): ART medications can be taken preventatively by HIV-negative individuals who are at substantial risk of HIV acquisition. PrEP is particularly recommended for individuals with HIV-positive partners and others engaging in high-risk behaviors.
Care for Children with HIV: Ensuring that children living with HIV receive timely and consistent ART is essential for their healthy growth, overall development, and long-term well-being. Strict adherence to the prescribed ART regimen is crucial for treatment success in children.
Viral Load Reduction and Transmission Prevention: ART effectively reduces the amount of HIV in the body, often to undetectable levels. This viral suppression dramatically lowers the risk of onward HIV transmission, as well as improving the health of the treated individual.
Improved Quality of Life: Effective ART significantly enhances the quality of life for people living with HIV. By suppressing viral replication and strengthening the immune system, ART reduces the incidence of opportunistic infections and other debilitating HIV-related complications.
Increased Life Expectancy: Extensive evidence demonstrates that ART has revolutionized the prognosis for people with HIV, substantially increasing their life expectancy and enabling them to live longer and healthier lives.
Prevention of Sexual HIV Transmission (Treatment as Prevention – TasP): By achieving and maintaining an undetectable viral load through ART, individuals living with HIV effectively eliminate the risk of sexually transmitting the virus to their partners. This “treatment as prevention” strategy is a cornerstone of HIV prevention efforts.
Reduced HIV-Related Stigma and Discrimination: Successful ART outcomes contribute to reducing stigma and discrimination associated with HIV. As individuals on ART live healthy, productive lives, public perceptions of HIV can shift, fostering greater understanding and acceptance.
Management of HIV Co-infections: ART plays an important role in the overall management of individuals with HIV, including the concurrent management of common co-infections such as hepatitis B and C, tuberculosis, and other conditions that are frequently observed in people living with HIV.
HIV/AIDS Prevention
In Uganda, the HIV epidemic is a complex issue driven by a combination of behavioral, biomedical, and structural factors. Consequently, no single HIV prevention strategy is sufficient on its own to prevent all new HIV transmissions. A multi-faceted approach is essential.
Behavioral Change and Risk Reduction Strategies
Promoting behavioral changes that reduce HIV risk is a critical component of prevention efforts. These strategies include:
Encouraging delayed initiation of sexual activity, particularly among young people.
Promoting safer sexual practices, including consistent and correct condom use.
Discouraging intergenerational sexual relationships, which are associated with increased HIV risk.
Types of Behavioral Change Interventions:
Service Delivery Optimization: Ensuring effective service delivery through dedicated focal persons, comprehensive staff training programs, and proactive community outreach initiatives to improve access to prevention services.
Risk Assessment and Counseling: Offering routine HIV testing services coupled with thorough assessments of individual sexual behaviors and risk profiles. Providing targeted Socio-Behavioral Change Communication (SBCC) tailored to individual needs identified through risk assessment.
Condom Promotion and Education: Actively promoting consistent and correct condom use as a key prevention method. This involves addressing common misconceptions about condoms, overcoming barriers to condom use, and ensuring widespread access to condoms.
Biomedical Prevention Interventions
A range of biomedical interventions play a crucial role in preventing HIV transmission:
Elimination of Mother-to-Child Transmission (eMTCT): eMTCT programs are specifically designed to prevent the vertical transmission of HIV from mothers to their infants during pregnancy, childbirth, and breastfeeding. These programs encompass a comprehensive package of interventions, including ART for pregnant women, safe delivery practices, and infant prophylaxis.
Safe Male Circumcision (SMC): Numerous studies have demonstrated that voluntary medical male circumcision (SMC) significantly reduces the risk of heterosexual HIV acquisition in men by approximately 60%. SMC also offers additional health benefits, including reducing the risk of acquiring other sexually transmitted infections (STIs) such as human papillomavirus (HPV) and herpes simplex virus type 2 (HSV-2).
Antiretroviral Therapy (ART) for Prevention (TasP): As described earlier, ART is a powerful prevention tool. By effectively suppressing the viral load in people living with HIV to undetectable levels, ART virtually eliminates the risk of sexual HIV transmission to their partners. This “Treatment as Prevention” (TasP) strategy is a cornerstone of current HIV prevention efforts.
Post-Exposure Prophylaxis (PEP): PEP involves the emergency use of antiretroviral medications following a potential HIV exposure event to prevent infection from taking hold. PEP must be initiated within a 72-hour window after exposure to be effective and typically involves a 28-day course of ART drugs. Common scenarios for PEP use include occupational exposures (e.g., needlestick injuries in healthcare settings) and non-occupational exposures (e.g., unprotected sexual encounters, sexual assault).
Pre-Exposure Prophylaxis (PrEP): PrEP is the regular, daily use of antiretroviral medication by HIV-negative individuals to significantly reduce their risk of acquiring HIV infection. PrEP is a highly effective prevention method for individuals at substantial risk, including those with HIV-positive partners, people who inject drugs, and individuals with high-risk sexual behaviors. When taken consistently as prescribed, PrEP reduces the risk of HIV acquisition from sexual contact by approximately 99% and from injection drug use by at least 74%.
Blood Transfusion Safety: Ensuring the safety of blood and blood products is paramount to prevent transfusion-transmitted HIV. This is achieved through rigorous screening of all blood donors for HIV and other bloodborne pathogens. Strict protocols are implemented for every stage of blood handling, from collection and testing to storage and transfusion, to minimize any risk of contamination.
STI Screening and Treatment: Regular screening for and prompt treatment of sexually transmitted infections (STIs) are essential components of HIV prevention. The presence of STIs can significantly increase both the susceptibility to HIV infection and the likelihood of HIV transmission if infection occurs. Public health efforts emphasize routine health check-ups and STI screenings, particularly for individuals in high-risk populations. Providing timely and effective treatment for any detected STIs is crucial for reducing STI-related complications and minimizing onward HIV transmission risk.
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved Design & Developed by Opensigma.co

