Terms in used research
Table of Contents
Common terms used in Research
Abstract: A brief, comprehensive summary of a research study. It conveys the most crucial aspects of the research, allowing readers to quickly grasp the study’s essence.
Assumption: A statement accepted as true or valid without needing formal proof. It’s based on logic or prior knowledge and is taken for granted as a starting point for research.
Data: Individual pieces of information collected for analysis. These are the raw facts and figures that form the basis of research findings.
Descriptive Research: A type of non-experimental research aimed at exploring and detailing new areas of knowledge. It’s used when little is known about a particular phenomenon, seeking to uncover initial insights and meanings.
Hypothesis: A testable statement predicting a relationship between two or more variables in a study. It’s an educated guess or proposed explanation that researchers aim to examine and either support or refute through their research.
Informed Consent: A voluntary agreement from a research participant to take part in a study. This agreement is given after the participant has received complete and understandable information about the study, including its purpose, procedures, risks, and benefits.
Phenomena: Observable facts, occurrences, or events that can be experienced and described through our senses, rather than solely through abstract thought or intuition.
Reliability: The consistency and stability of a measurement tool over time. It indicates the degree to which a measurement is free from random errors and produces consistent results when repeated.
Validity: The extent to which a measurement tool accurately measures what it is intended to measure. It reflects how truthfully the research findings represent the real-world phenomenon being studied.
Variable: A characteristic or attribute that can take on different values. Examples include height, weight, age, or opinions, as these can vary from person to person or situation to situation.
Dependent Variable: The variable that is expected to be influenced or changed as a result of manipulating the independent variable. It’s the outcome or effect being measured.
Independent Variable: The variable that is intentionally manipulated or changed by the researcher to observe its effect on the dependent variable. It’s considered the potential cause.
Confounding Variable: An extraneous variable that is not the focus of the study but could potentially influence the results, creating misleading or unclear findings if not controlled for.
Qualitative Data: Descriptive data characterized by words, narratives, observations, or images. It focuses on understanding qualities, meanings, and experiences rather than numerical measurements.
Quantitative Data: Numerical data that can be counted, measured, and expressed as numbers. It is often used for statistical analysis and focuses on quantities and amounts.
Population: The entire group of individuals, objects, or events that meet specific criteria of interest to the researcher and to whom the research findings are intended to apply.
Sample: A smaller, manageable subgroup selected from the population to represent the characteristics of the entire population. Data is collected from the sample and used to draw inferences about the larger population.
More Terminologies in Simple Detail
ABSTRACT
A concise and clear summary of a research paper, typically found at the beginning of scholarly articles. It serves two main purposes:
Relevance Assessment: To quickly help potential readers decide if the paper is relevant to their own research interests, saving them time.
Key Findings Communication: To communicate the most important findings of the research to those who may not have time to read the entire paper in detail.
DATA
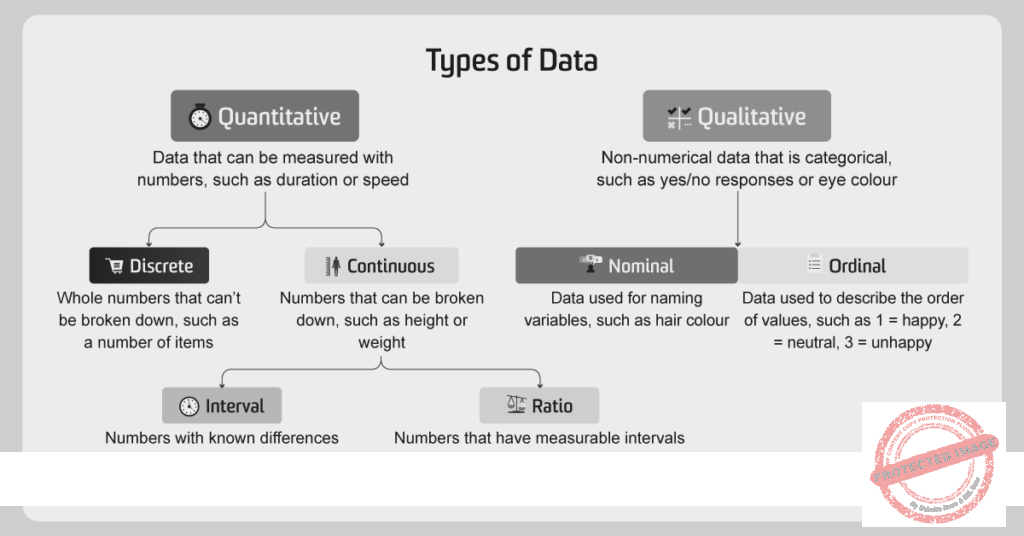
Data represents units of information, encompassing statistics, factual details, numerical figures, general observations, evidence, or insights gathered throughout a research study. It is essentially the processed information collected. Data is broadly categorized into four main scales or types:
Nominal Data
Ordinal Data
Discrete Data
Continuous Data
These classifications or scales further define the nature and characteristics of data in research.
VARIABLES
Definition: Variables are characteristics or attributes that can take on different values. They are qualities, quantities, or properties of people, objects, or situations that are subject to change or variation.
Explanation: Think of variables as the things you are measuring or studying that can differ. For example, age, gender, income, and test scores are all variables because they can have different values for different individuals or in different situations.
INDEPENDENT VARIABLE
Definition: The independent variable is the variable that a researcher deliberately manipulates or changes. It is also known as the “manipulated variable.”
Explanation: This is the variable that is considered the potential cause in a cause-and-effect relationship. In the example “factors influencing the uptake of family planning services,” “factors influencing” represents the independent variable because these factors are what might be affecting the uptake.
DEPENDENT VARIABLE
Definition: The dependent variable is the variable that is expected to be affected or influenced by the independent variable. It is also referred to as the “outcome variable.”
Explanation: This is the variable that is considered the potential effect or outcome. Researchers are interested in understanding, explaining, or predicting changes in the dependent variable. In the “family planning uptake” example, “uptake of family planning services” is the dependent variable, as it is what the researchers are trying to understand and potentially influence through other factors.
OPERATIONAL DEFINITION
Definition: An operational definition specifies exactly how a researcher will measure the variables they are investigating. It is a clear and precise description of how a variable will be observed and quantified in the actual study.
Explanation: It’s about making abstract concepts measurable. For example, if you are studying “stress,” you need an operational definition of how you will measure stress – will it be through a questionnaire, physiological measures like heart rate, or observations of behavior? The operational definition makes the variable concrete for your research.
HYPOTHESIS
Definition: A hypothesis is a statement that predicts a relationship between two or more variables in a research study. It is an educated or reasoned guess formulated by the researcher.
Explanation: A hypothesis is a testable prediction. It’s a researcher’s best guess about what they expect to find in their study, based on existing knowledge or theory. Research is then conducted to see if the data supports or refutes the hypothesis.
LIMITATIONS
Definition: Limitations are restrictions or weaknesses within a research study that may affect the credibility and how widely the findings can be applied (generalizability). These are potential flaws or shortcomings in the research.
Explanation: Limitations can arise from various sources, such as limited resources, a small sample size, methodological weaknesses, or other factors beyond the researcher’s complete control. Acknowledging limitations demonstrates research integrity and a thorough understanding of the study’s context.
POPULATION
Definition: In research, a population refers to the entire group of individuals or objects that share certain characteristics and are of interest for a particular study.
Explanation: The population is the complete set of cases that the researcher wants to learn about. It could be all people with a specific disease, all hospitals in a region, or any defined group that fits the study’s criteria.
TARGET POPULATION
Definition: The target population is the entire population that researchers are interested in drawing conclusions about and to which they intend to generalize their research findings.
Explanation: This is the ideal population that the researcher wants to study and make inferences about. It’s the larger group that the research seeks to understand.
ACCESSIBLE POPULATION
Definition: The accessible population is the subset of the target population that is actually available to the researcher for a particular study.
Explanation: In practice, it’s often impossible to study the entire target population. The accessible population is the portion of the target population that is realistic and feasible for the researcher to reach and recruit for their study.
SAMPLE
Definition: A sample is a smaller, selected portion or subset of the population that is chosen to participate in a research study.
Explanation: Researchers study samples to gather data that will represent the larger population. Studying a sample is more practical, cost-effective, and time-efficient than studying the entire population.
REPRESENTATIVE SAMPLE
Definition: A representative sample is a sample that closely mirrors the characteristics of the population from which it was drawn.
Explanation: The goal of sampling is to obtain a sample that is representative of the population so that findings from the sample can be generalized back to the larger population with confidence.
SAMPLING
Definition: Sampling is the act, process, or technique of selecting a representative portion (sample) from a population to determine characteristics of the whole population. It is the method used to choose participants for a study.
Explanation: Sampling is the systematic process of selecting a subset of individuals or units from a population. Effective sampling is crucial for ensuring that the research findings are generalizable to the population of interest.
PROBABILITY SAMPLING
Definition: Probability sampling involves selecting subjects or sampling units from a population using a random procedure.
Examples: Simple Random Sampling, Stratified Random Sampling
Explanation: In probability sampling, every member of the population has a known, non-zero chance of being selected into the sample. This randomness helps to minimize bias and increase the likelihood of obtaining a representative sample.
NON-PROBABILITY SAMPLING
Definition: Non-probability sampling involves selecting subjects or sampling units from a population using a non-random procedure.
Examples: Convenience Sampling, Purposive Sampling
Explanation: In non-probability sampling, selection is not based on random chance. While easier to implement, non-probability sampling methods are more prone to bias and may limit the generalizability of findings to the larger population.
RELIABILITY
Definition: Reliability refers to the degree of consistency and accuracy with which a measurement instrument measures the attribute it is designed to measure.
Explanation: A reliable instrument produces similar results when used repeatedly under similar conditions. It is about the consistency and dependability of the measurement.
VALIDITY
Definition: Validity is the degree to which a measurement instrument truly measures what it is intended to measure.
Explanation: A valid instrument accurately reflects the concept or construct it is designed to assess. It is about the accuracy and truthfulness of the measurement.
PRE-TESTING
Definition: Pre-testing is a stage in research where data collection instruments, such as questionnaires, are tested with a small group of individuals from the target population before the main study.
Explanation: It’s a trial run of your data collection tools. By administering instruments to a smaller group, researchers can identify and resolve potential issues before the full-scale data collection begins.
Purpose of Pretesting
Problem Identification: To find and fix problems with the data collection instrument, such as unclear questions, confusing wording, or formatting issues.
Validity Assurance: To ensure that the data collection tools are valid and measure what they are intended to measure, leading to more reliable results.
Feasibility Assessment: To determine if respondents are able and willing to provide the necessary information, and to check if the questions are appropriate and understandable for the target population.
Solution Testing: To allow researchers to test potential solutions to identified problems and refine the questionnaire before wider use.
Principles of Pretesting
Realistic Conditions: Pre-testing should be conducted under conditions that closely resemble the actual data collection environment.
Representative Participants: Participants in the pre-test should be similar to those who will be included in the main study sample in terms of their characteristics.
Detailed Note-Taking: Careful notes should be made about any problems encountered during pre-testing, along with potential solutions identified.
PILOT STUDY
Definition: A pilot study is a smaller, preliminary version of the main research study. It is conducted to refine the methodology and test the feasibility of the research design.
Explanation: A pilot study is like a dress rehearsal for the main study. It involves using similar subjects, settings, procedures, data collection methods, and analysis techniques as planned for the full study, but on a smaller scale.
Purpose of Pilot Study
Methodology Refinement: To explore and test research elements and make necessary modifications to research tools and methodology before the main study.
Feasibility Assessment: To ascertain the practicality and feasibility of conducting the full-scale research project, including data collection instruments and procedures.
ANALYSIS
Definition: Analysis is the systematic process of organizing, structuring, and examining data in order to answer research questions or draw meaningful conclusions.
Explanation: Data analysis is the critical step where raw data is transformed into interpretable findings. It involves using various techniques to identify patterns, relationships, and insights within the data. Effective analysis is essential for drawing valid and reliable conclusions from research. Data analysis typically follows the presentation and interpretation of research findings.
INFORMED CONSENT
Definition: Informed consent is a process where individuals learn key facts about a research study, particularly a clinical trial, before deciding whether to participate. It is an ongoing process of providing information throughout the study.
Explanation: Informed consent is a cornerstone of ethical research involving human participants. It ensures that individuals have sufficient information to make a voluntary and informed decision about whether to participate. Researchers must explain the study details to potential participants, and consent must be freely given and continuously respected.
COHORT
Definition: In epidemiology, a cohort is a defined group of individuals who share a common characteristic or experience.
Types: Cohorts can be prospective (followed forward in time from the present to the future) or retrospective (studied based on past data and experiences).
Explanation: Cohort studies are a type of observational research that follows groups of individuals (cohorts) over time to examine the occurrence of outcomes, like diseases, in relation to exposures or characteristics they share.
BIAS
Definition: Bias occurs when a particular viewpoint or influence prevents impartial or objective judgment on issues related to a subject.
Explanation: Bias can systematically distort research findings, leading to inaccurate or misleading conclusions. In clinical studies, techniques like blinding and randomization are used to minimize and control bias.
BLIND (or Masked)
Definition: In a “blind” or “masked” randomized trial, participants are not told which treatment group (or arm of the trial) they are assigned to.
Explanation: Blinding is a technique used to reduce bias, particularly in clinical trials. By keeping participants (and sometimes researchers) unaware of treatment assignments, it helps to prevent expectations or knowledge of treatment from influencing outcomes or reporting.
SIDE EFFECTS
Definition: Side effects are any unintended or undesired actions or effects of a drug or treatment.
Explanation: Side effects can range from minor and temporary to serious and long-lasting. They are a critical consideration in medical research and treatment development. Experimental drugs and treatments must be thoroughly evaluated for both immediate and long-term side effects to ensure patient safety.
Get in Touch
(+256) 790 036 252
(+256) 748 324 644
Info@nursesonlinediscussion.com
Kampala ,Uganda
© 2025 Nurses online discussion. All Rights Reserved Design & Developed by Opensigma.co

